1. Background
Sexually transmitted infections (STIs) represent an escalating public health issue. Despite their largely preventable nature, they pose a significant challenge (1), leading to a spectrum of negative outcomes on both individual and public health, ranging from mild and acute conditions to severe and chronic complications (2). In addition to substantial morbidity and mortality, STIs inflict considerable socio-economic burdens (3).
Accurately quantifying the prevalence and incidence of STIs is essential for understanding the epidemiology of these diseases, which includes surveillance, prevention, and treatment efforts. Many countries rely on routine case reporting systems, while others utilize sentinel sites to estimate these indices (4).
The establishment of an STI surveillance system aims to provide reliable estimates of crucial epidemiological indices and monitor their trends over time (5). Although routine registration and reporting systems can act as effective early warning mechanisms in areas lacking information on waterborne infections (6, 7), the accuracy of the estimated indices remains a significant concern, even for diseases with more robust reporting systems (8).
The cornerstone of any surveillance system involves its registration and reporting procedures (9). In the context of STIs, comprehending the diseases' epidemiology extends well beyond merely diagnosing and treating them (10). It necessitates a deeper understanding of the dynamics of infections and the social interactions of patients within core groups in the community (7).
The participation of numerous service providers in both urban and rural populations, including public and private clinics, hospitals, and pharmacies, in diagnosing and treating STI patients poses a significant challenge to the validity and reliability of surveillance systems that operate based on routine registry databases (11).
In the study by Ahmadnia et al. (12), the prevalence of urinary infections among married women in Zanjan was estimated to be 4.7%. Most studies conducted in Iran to explore the prevalence of infections have typically focused on at-risk groups (13, 14). These studies were not carried out on the general population, nor were they conducted randomly.
2. Objectives
The aim of this study was to assess the completeness and representativeness of the current Iranian STI surveillance system and to propose correction coefficients to enhance the validity of the existing metrics.
3. Methods
3.1. Settings
The STI surveillance system in Iran was initiated in 1998 and underwent revision in 2006. Its primary functions include assessing the prevalence of STIs in various groups (such as pregnant women) and the routine reporting of cases. This population-based cross-sectional study was conducted in 2018 on a random sample of 3,879 participants aged 18 - 50 from both the rural and urban populations of Marvdasht, a large county in Iran. Marvdasht is the second largest and most populous county in Fars province, located 45 km north of Shiraz, the province's capital. The county has a population of approximately 221,163, with 148,858 residing in urban areas and 72,305 in rural areas. The socio-economic status of Marvdasht, being both an industrial and agricultural area, contributes to its multicultural and multi-ethnic population, making it an excellent representation of Iranian cultural and social characteristics (9, 12).
3.2. Selection of Participants
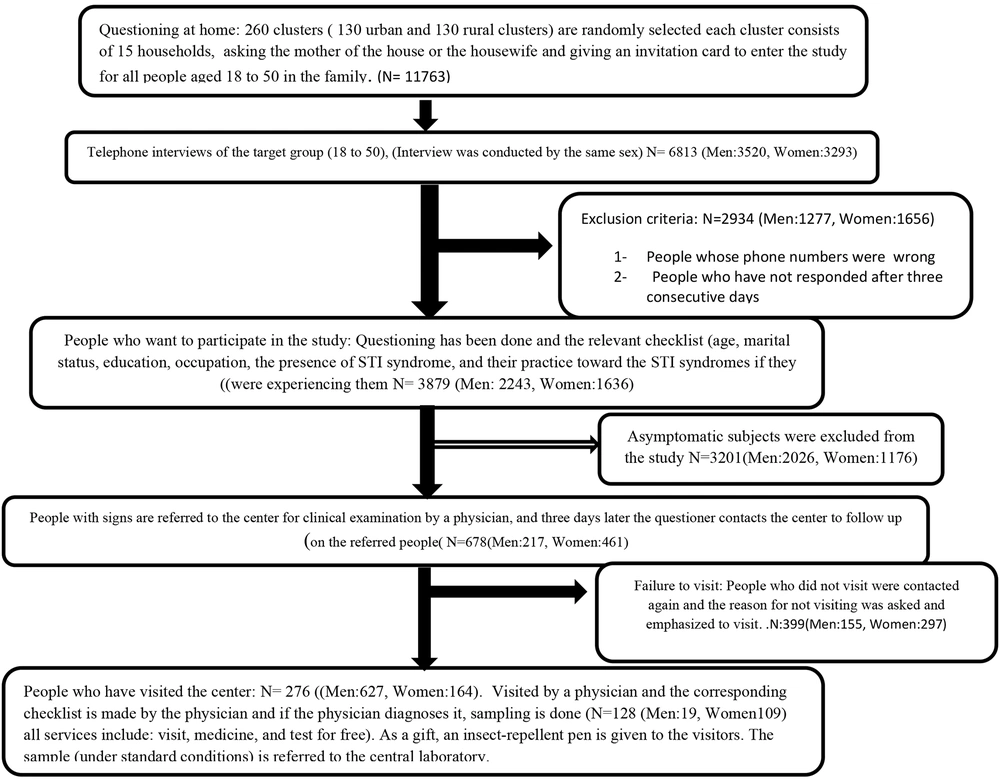
For the selection of participants, the first 260 clusters—comprising 130 urban and 130 rural clusters—were randomly chosen. Each cluster consisted of 15 households, and individuals aged 18 - 50 years were surveyed in each household.
3.3. Definition of a Cluster
According to the health survey plan by the Ministry of Health and Medicine, a cluster in this study was defined by assigning a specific number to each household at the base level, utilizing household file information. Subsequently, the households were sequentially listed, and from this list, 130 households were selected as the "head of the cluster." Following this selection, 15 households to the right of each "head of the cluster" were surveyed. In the rural phase, all the villages covered by the study were listed within the city, with each village being assigned a unique number. From this list, 130 villages (numbers) were randomly chosen. In each of these 130 villages, all household file numbers were cataloged, and one family was randomly selected as the head of the cluster.
The phone interviews were conducted by trained interviewers of the same sex as the participant (male or female), beginning with a concise explanation of the study's objectives and providing participants with further assurances regarding the confidentiality of their information. After the brief introduction, participants were informed that the interview could be terminated at any moment upon their request. Additionally, verbal consent was obtained from the participants over the phone.
In this study, three checklists were utilized. The first checklist was general and was completed at home by questioning the mother or the head of the household. The second checklist focused on the person's behavior at the time of the syndromes and was filled out through telephone inquiries. The third checklist was completed in person by a urologist and gynecologist for individuals who had exhibited symptoms in the previous stage (Figure 1).
3.4. Statistical Analysis and Calculation of the Correction Factor
Among the several syndromes identified by the Iranian Ministry of Health, we utilized genital ulcers and secretions in both genders as the primary outcomes to assess the system's completeness and to calculate the correction factor. We developed a mathematical model to determine the correction factor for estimating the prevalence of the selected syndromes in each gender. This model takes into account all factors that influence the registration and reporting of cases, as well as the latent period for the cases. Employing the "backward calculation method," we utilized both the registered cases and the cases detected in the survey for the calculations (Table 1 and Equation 1). The correction factor was estimated by dividing the cases projected from the mathematical model by the cases reported by the surveillance systems.
| Notation of Parameters | Model | Description | Source of Data | Urethral Discharge Men/Women | Genital Ulcer Men/Women |
|---|---|---|---|---|---|
| Ps | 1 | Reported prevalence based on registered data | Routine STI case reporting | 0.09/0.12 | 0.04/0.19 |
| pc | 1 | The possibility of seeking treatment in people with sexual infections in Iran | Present study | 62.3/92.5 | 62.3/92.5 |
| pC1 | 1 | The possibility of the patient going to the public sector | Present study | 38.3/38.3 | 38.3/38.3 |
| PC2 | 1 | The possibility of the patient going to the private sector | Present study | 58.7/60.6 | 58.7/60.6 |
| Pp1 | 1 | The possibility of the participation of the public sector in the reporting of sexually transmitted infections | Present study | 53.4/53.4 | 53.4/53.4 |
| PP2 | 1 | The possibility of private sector participation in reporting sexually transmitted infections | Present study | 9.8/9.8 | 9.8/9.8 |
| Pu | 1 | A share of visited patients who are not reported | Literature review | 75.7/56.8 | 75.7/56.8 |
| SEcd | 1 | Sensitivity of clinical diagnosis of each infection | Literature Review | 97.5/98 | 97.5/98 |
| SPcd | 1 | Specificity of Clinical diagnosis feature of each infection | Literature review | 98/75 | 98/75 |
| Me | 1 | The average number of occurrences of infection or syndrome | Literature review/WHO guideline | 2.5/2.5 | 3.5/3.5 |
Abbreviation: STI, sexually transmitted infection.
3.5. Statistical Analysis
STATA 13.1 was used for data analysis.
3.6. Ethical Approval
The protocol for this study received approval from the ethical committee of Shiraz University of Medical Sciences (reference code: IR.SUMS.REC.131096).
4. Results
A total of 3 879 individuals aged 18 - 50 participated in this study. The majority of the participants were male (57.89%), lived in urban areas (53.62%), and were married (69.63%). The age group under 30 years exhibited the highest frequency (39.95%) among the groups (Table 2).
| Variables | Values a |
|---|---|
| Sex | |
| Male | 2243 (57.89) |
| Female | 1636 (42.17) |
| Residency | |
| Urban | 2080 (53.62) |
| Rural | 1799 (46.37) |
| Marital status | |
| Single | 1144 (29.49) |
| Married | 2701 (69.63) |
| Job | |
| Worker/free | 1308 (35.25) |
| Employee | 251 (6.76) |
| Farmer | 298 (8.03) |
| Housewife/unemployed | 1578 (42.53) |
| Soldier/student/retired | 275 (7.41) |
| Education | |
| Literate/primary | 951 (24.515) |
| Secondary/high school | 2104 (54.24) |
| Academic | 819 (21.11) |
| Age | |
| < 30 | 1550 (39.95) |
| 31 - 40 | 1377 (35.49) |
| 41 - 50 | 952 (24.54) |
| Having any kind of sexual contact | |
| Yes | 2714 (69.96) |
| No | 1165 (30.03) |
| Age | 34.28 ± 8.85 |
a Values are expressed as No. (%) or mean ± SD.
The observed prevalence of urethral discharge in men and women is 25.32% (23.08 - 27.56) and 47.03% (39.93 - 54.13), respectively. Regarding genital ulcers, the observed prevalence in men and women is 5.16% (4.06 - 6.86) and 15.50% (9.5 - 21.5), respectively (Table 3).
| Syndrome/Agent | Population-Based, % | Surveillance System, % | Completeness, % |
|---|---|---|---|
| Men | |||
| Urethral discharge | 4.84 (1.01 - 13.50) | 0.09 (0.07 -0.011) | 25.33 (23.08 - 27.56) |
| Genital ulcer | 0 | 0.04 (0.03 - 0.06) | 5.16 (4.06 - 6.86) |
| Women | |||
| Urethral discharge | 60.98 (53.06 - 68.49) | 0.12 (0.1 - 0.15) | 47.03 (39.93 - 54.13) |
| Genital ulcer | 11.59 (7.12 - 17.50) | 0.19 (0.16 - 0.22) | 15.50 (9.5 - 21.5) |
Table 4 shows the surveillance system's completeness for the selected symptoms by gender. The correction factor for urethral discharge is 124.7 for men and 7.26 for women. The prevalence of urethral discharge and genital ulcers in men is 11.16% (10.95 - 11.38) and 3.11% (2.99 - 3.23), respectively, and in women are 10.63% (10.42 - 10.84) and 1.41% (1.33 - 1.49), respectively.
| Syndrome/Agent | Routine STI Case Reporting | Correction Ratio | Population Corrected Cases | Prevalence, % |
|---|---|---|---|---|
| Men | ||||
| Urethral discharge | 74 | 124.7 | 9230 | 11.16 (10.95 - 11.38) |
| Genital Ulcer | 35 | 73.37 | 2568 | 3.11 (2.99 - 3.23) |
| Women | ||||
| Urethral discharge | 95 | 89.41 | 8494 | 10.63 (10.42 - 10.84) |
| Genital Ulcer | 155 | 7.26 | 1126 | 1.41 (1.33 - 1.49) |
Abbreviation: STI: sexually transmitted infection.
5. Discussion
The aim of this study was to evaluate the current syndromic surveillance system for STIs by assessing the completeness and representativeness of the system. Additionally, we provided a correction factor to adjust the reported prevalence of the selected syndromes.
The findings indicated that the prevalence reported by the health system is significantly underestimated in both genders. Generally, only 25 - 38% of medical practitioners who are required to report cases of STIs do so on a monthly basis (15). The lack of participation from physicians is even more pronounced in the private sector than in the public sector, leading to underreporting, particularly among men who prefer the private sector (16). This underestimation is attributed to factors such as lack of motivation, insufficient knowledge among physicians about the importance of reporting STI cases, lack of feedback, conflicts of interest, and reluctance to report STIs, resulting in a considerable number of patients who seek care in the private sector not being registered and reported to the system (13).
In Iran, pharmacists, physicians, midwives, and laboratories are required to report STIs based on defined syndromes in both sexes. Various studies have indicated that the syndromic diagnosis of STIs, such as genital ulcers and urinary tract secretions, lacks satisfactory validity due to low sensitivity and specificity. Consequently, we incorporated a parameter in the model to account for the sensitivity of symptom-based diagnosis of STIs (16).
In men, the completeness of the national surveillance system for genital ulcers was found to be surprisingly low. As previously discussed, most individuals with STI syndromes tend to seek care in the private sector due to the importance of confidentiality and the stigma associated with STIs (17). Considering that sex outside of marriage is prohibited in Iran, individuals with STIs face significant stigma, which may deter them from seeking treatment. This contributes to a severe underestimation of the prevalence of STI symptoms in the population, especially among men, as the prevalence of risky behaviors is higher in Iranian men than in women (18).
The prevalence of genital discharge in women is relatively high and significantly differs from the data recorded in the surveillance system. This discrepancy can be attributed to factors such as social stigma, family conflicts, lack of trust, dissatisfaction with the services, and the embarrassment of being examined by a doctor. Consequently, women may delay seeking treatment, hoping for symptoms to improve on their own, or resort to self-medication as they can purchase medicines from a pharmacy without a prescription (19, 20). This issue may also be related to the low sensitivity and specificity of certain symptoms associated with STIs, particularly in women. Some symptoms may overlap with those of other diseases, meaning the presence of a specific symptom does not necessarily indicate an STI (21, 22).
The number of cases of syndromes and STIs estimated in the model for the population was significantly higher than those recorded in the care system for STIs. This discrepancy varied according to the type of syndrome, with the greatest difference observed in discharge cases in men and the smallest difference in ulcer cases in women. The disparity between the estimated cases at the disease center and elsewhere highlights a deficiency in the current registration and reporting system for STIs and indicates a significant underestimation of these infections.
Consequently, by comparing the number of syndrome cases identified in the study with those reported in the system, we calculated a coefficient to adjust the cases recorded in the registration system. This correction factor can be utilized to more accurately estimate the actual number of disease cases in the population.
5.1. Limitations
(1) The estimated median for the number of events per syndrome or infection is assumed to be uniform across all age groups. However, it is likely that this number varies with age.
(2) The incidence of STIs among groups with high-risk sexual behaviors, such as sex workers, is probably underestimated. In this study, we assumed that all information for these groups was completely and accurately recorded in the current registration and reporting system. Consequently, we did not apply the correction factor in the model to account for the impact of cases in these groups on the estimated prevalence and incidence of STIs in the country.
(3) The model was treated as static, disregarding the dynamics of the variables over time. It was assumed that all model variables remained constant throughout the year and did not change. The assumption extended to demographic shifts, suggesting individuals do not transition between age groups. Additionally, model calculations were performed for a single point in time (2019) to estimate incidence based on point prevalence without removing individuals previously infected from the denominator.
(4) This issue may stem from the low sensitivity and specificity of certain symptoms associated with STIs, particularly in women. Some symptoms may overlap with those of other diseases, meaning the presence of a specific symptom does not conclusively indicate an STI (21, 22).
5.2. Conclusions
The estimated prevalence of syndromes related to STIs, as determined in this study (using both the population and the model), is significant. Furthermore, the findings reveal an underestimation of the reporting system's data. By tracing the journey from the onset of an individual's infection to its registration in the reporting system, a correction factor can be applied to the data reported in each department to achieve a more accurate estimate. Additionally, establishing an observation base in each city could enable the estimation of the incidence and prevalence of these infections in the same population while considering cultural and social factors.