1. Background
Diabetes mellitus is a well-known metabolic disorder characterized by an abnormality in glucose homeostasis. The deregulation of glucose metabolism results in increased blood glucose levels, leading to long-term complications in many organs (1). The liver plays a critical role in the synthesis and metabolism of carbohydrates, lipids, and proteins. Previous studies have reported that diabetes causes various forms of liver damage, including abnormal glycogen accumulation, nonalcoholic fatty liver, and cirrhosis (2, 3). The exact mechanisms that lead to liver damage in diabetic patients are not fully understood. Several mechanisms are involved in liver injury in diabetic subjects.
First, hyperglycemia induces oxidative stress and inflammatory responses due to the activation of Kupffer cells and the production of free radicals. Second, reactive oxygen species (ROS) stimulate the release of inflammatory factors, leading to the infiltration of leukocytes and the induction of adhesion molecules. Third, ROS can destroy liver tissue by stimulating apoptosis in hepatocytes. Finally, the levels of reduced glutathione (GSH), a crucial antioxidant, decrease in diabetes, causing an elevation in oxidative stress products such as lipid peroxidation and malondialdehyde (MDA), a typical marker of oxidative stress (4). Despite significant success in using chemical drugs for the management of diabetes, many traditional plants have been used throughout the world to mitigate diabetes complications and aid in its treatment due to the adverse effects of chemical drugs (5). In recent years, traditional plants have attracted significant attention as anti-diabetic agents (6). One such plant, known for its abundance of effective phytochemical components and its traditional use in diabetes treatment, is cinnamon. Cinnamon is obtained from the tropical evergreen cinnamon plant and comes in two main types: Cassia cinnamon (C. aromaticum and C. burmannii) and Ceylon cinnamon (Cinnamomum verum and C. zeylanicum) (7). While cinnamon has commonly been used as a dietary supplement, recent research has discovered its antioxidant, anti-inflammatory, anti-obesity, and anti-hyperlipidemia properties (5, 8). Previous studies indicate that cinnamon has beneficial effects on glucose utilization and hyperlipidemia (9, 10). Much attention has been paid to cinnamon as a potential drug for preventing diabetes complications over the past 30 years. Numerous experiments have suggested that cinnamon affects blood glucose levels and insulin signal transduction (11-14). Due to its many antioxidant agents—cinnamaldehyde, cinnamic acid, eugenol, coumarin, and procyanidins—cinnamon can scavenge free radicals and reduce lipid peroxidation and MDA formation (2, 15, 16).
Cinnamon exerts its antidiabetic effects through several mechanisms: (A) Inhibiting intestinal glucose absorption and enhancing cellular glucose uptake, (B) enhancing glycolysis and glycogenesis processes while suppressing gluconeogenesis, and (C) stimulating insulin secretion and enhancing insulin receptor activity (17). Dou and colleagues claimed that the antidiabetic characteristics of cinnamon are attributed to the presence of methyl hydroxy chalcone polymer (MHCP), which effectively mimics insulin effects. Methyl hydroxy chalcone polymer can stimulate insulin production by activating glycogen synthase and inhibiting glycogen synthase kinase-3β activity (18).
A previous meta-analysis study reported that cinnamon significantly decreased fasting blood glucose (FBG) and the homeostatic model assessment for insulin resistance (HOMA-IR) levels compared to a placebo (19). In addition, Rao and Gan reported that the aqueous extract of cinnamon substantially inhibited the absorption of alanine in the rat intestine. Alanine is converted back to pyruvate in the liver and is used as a substrate for gluconeogenesis (20). Most studies in the field of diabetes have focused on evaluating the effects of cinnamon extract (CE) on glucose and antioxidant levels in serum, but the effects of CE on antioxidant gene expression in the liver have not been investigated. Although many reports exist, most are restricted to the positive effects of CE on diabetic models, and the adverse effects of CE on tissues, including the liver, have not been studied yet.
2. Objectives
The objective of this study was to investigate the potential protective properties of Cinnamon zeylanicum extract against glucose levels, oxidative stress indicators, antioxidant enzyme gene expression, and liver histopathological changes in STZ-induced diabetic rats. The findings of this study may open new avenues for reducing complications and improving the treatment of this chronic disease.
3. Methods
3.1. Materials
Streptozotocin (STZ), 2-thiobarbituric acid (TBA), reduced glutathione (GSH), tetraethoxypropane (TEP), 5,5´-dithiobis-(2-nitrobenzoic acid) (DTNB), and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (St. Louis, MO). Primers were provided by Arian Gene (Iran), and a One-step SYBR PrimeScript real-time PCR kit was purchased from Takara Company (TaKaRa, Japan). An enzymatic kit (glucose oxidase) for the determination of glucose concentration was prepared by Pars Azmoon Company (Tehran, Iran). Cinnamon and other common reagents were purchased from commercial sources in Iran.
3.2. Animals
Forty adult male Sprague Dawley rats weighing 250 - 300 g were included in this study. The animals were purchased from the animal house of Shiraz University of Medical Sciences between August 2021 and October 2021. The rats were randomly allocated into four groups using a random number table. Each pair of rats was kept in a separate cage and housed at room temperature with a 12-hour light/dark cycle and 50% relative humidity. They were provided with standard food and water ad libitum.
3.3. Experimental Design
Diabetes was experimentally induced in rats with a single intraperitoneal (i.p.) injection of streptozotocin (STZ) at a dosage of 50 mg/kg body weight after 12 hours of fasting. Groups I and IV received normal saline alone, while groups II and III received STZ intraperitoneally. Diabetes was confirmed three days post-injection by measuring the FBG concentration using a glucometer, selecting rats with FBG greater than 300 mg/dL as diabetic. Subsequently, the FBG levels and body weight of the rats were measured every two weeks.
The four groups of rats included in this study (10 rats per group) were as follows:
Healthy control (1): Healthy control rats were given only distilled water daily by gavage.
Diabetic control (2): Diabetic control rats were given only distilled water daily by gavage.
Diabetic + Cinnamon (3): Diabetic rats received CE daily by gavage.
Healthy control + cinnamon (4): Healthy control rats received CE daily by gavage.
Rats in all groups were euthanized with a high dose of ether after eight weeks. Blood was collected by heart puncture, and plasma was isolated. The liver tissue of each rat was dissected and immediately frozen in liquid nitrogen for RNA extraction and biochemical analysis. Additionally, a piece of liver tissue was soaked in formalin for histopathological study.
3.4. Plant Material and Preparation of the Aquatic Extract of Cinnamon Zeylanicum
Cinnamon bark was collected from a local market in Shiraz (Fars province, Iran) and authenticated as cinnamon zeylanicum (CZ) by a botanist from the School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran. The voucher number for CZ with the code “PM 985” was deposited at the Department of Botany, Faculty of Pharmacy, Shiraz University of Medical Sciences.
The cinnamon extract CE was prepared according to previous reports (21). Briefly, 10 grams of cinnamon were powdered in a grinder and mixed with 100 mL of distilled water. The mixture was then incubated for two hours in a water bath at 60°C and filtered using Whatman filter paper No. 1. The extract was stored at 4°C until used for feeding the rats. This extract was diluted 1: 10 with distilled water and used for rat treatment.
3.5. Determination of Plasma Glucose Concentration
For measuring plasma glucose after 8 weeks, plasma was isolated from the blood collected from the rats' hearts following 12 hours of fasting. The fasting blood glucose (FBG) levels were determined using a commercial kit based on the glucose oxidase method (22). Additionally, the FBG levels of the rats were measured weekly using a glucometer.
3.6. Preparation of Liver Homogenate
The same lobe of the rat's liver was removed, washed with cold normal saline solution (0.9% NaCl), and then snap-frozen in liquid nitrogen for estimating biochemical factors. Fifty milligrams of the liver were cut into small pieces and homogenized in phosphate buffer (50 mM, pH 7.4) using a homogenizer. The liver homogenate was centrifuged at 5000 RPM for 30 minutes at 4°C. The supernatant was utilized for the determination of MDA and GSH levels.
3.7. Determination of Malondialdehyde (MDA)
The MDA, a marker of lipid peroxidation in the liver, was determined using the colorimetric method reported by Khorsand et al. (23). Briefly, the sample was mixed with trichloroacetic acid and TBA solution. After boiling at 100°C for 20 minutes, the mixture was centrifuged at 5000 g for 15 minutes. The absorbance of the supernatant was measured using a UV/Visible spectrophotometer at 532 nm against a blank. The concentration of MDA in the liver homogenate was calculated using a standard curve of 1,1,3,3-tetraethoxypropane and expressed as nmole/100 mg tissue.
3.8. Determination of Glutathione (GSH)
The GSH concentration in the liver homogenate was assayed using a modified Tietz method (24). Briefly, 0.1 mL of the deproteinized sample was introduced into the reaction mixture, which comprised phosphate buffer (100 mM, pH = 7.4), EDTA (1 mM), and DTNB (1.5 mg/mL). The absorbance of the mixture was recorded at 412 nm against a blank. The GSH level in each sample was calculated using a GSH solution as a standard and reported as nmol/100 mg tissue.
3.9. Total RNA Isolation and qRT-PCR Analysis of Hepatic Antioxidant Enzymes
For the evaluation of catalase (CAT) and glutathione reductase (GR) gene expression, fifty milligrams of liver tissue were used for RNA extraction. Quantitative real-time PCR was performed using a One-step SYBR PrimeScript RT-PCR kit-II (TaKaRa, Japan) and a real-time thermal cycler (Rotor-Gene 6000, Qiagen). The primer sequences for CAT, GR, and β-actin genes are shown in Table 1.
| Gene and Primer Sequence | Accession | Amplicon Size (bp) |
|---|---|---|
| β-Actin | NM_031144.3 | 155 |
| F: 5’-AGGTCATCACTATCGGCAAT-3’ | ||
| R: 5’-TGTGTTGGCATAGAGGTCTT-3’ | ||
| CAT | NM_012520.2 | 162 |
| F: 5’-CGATATTACCAGATACTCCAAG-3’ | ||
| R: 5’-CAGTTACCATCTTCAGTGTAG-3’ | ||
| GR | NM_053906.2 | 228 |
| F: 5’-CTCACAGGTTAAGGAAGTAA-3’ | ||
| R: 5’-TTGACATTGGTATTCTGGAA-3’ |
Abbreviation: CAT, catalase; GR, glutathione reductase; NM, accession number; bp, base pair.
Total RNA from liver tissue was extracted using RNX-Plus solution (Cinnagen, Iran) according to the manufacturer’s instructions. The purity and quantity of the total RNA were determined using a NanoDrop 1000 spectrophotometer. To remove potential genomic DNA contamination, DNase I (ThermoFisher, Carlsbad, CA, USA) was added to RNA samples following the manufacturer's protocol.
For the One-step SYBR-green method, cDNA synthesis and qRT-PCR were performed from one μg of DNase-treated RNA in a total volume of 25 μL. The PCR amplification reaction contained 1 μL of RNA, 1 μL of each primer (forward and reverse, 10 μM), PCR master mix (12.5 μL), and RNase-free dH2O to a total volume of 25 μL. The qRT-PCR was performed on each gene using the following parameters: Stage 1: Reverse transcription hold (42°C for 5 min, 95°C for 10 s); stage 2: PCR reaction, 40 cycles (95°C for 5 s, 60°C for 30 s).
Relative changes in the expression levels of GR, CAT, and beta-actin genes, used as a housekeeping reference gene, were determined by the 2-ΔΔCt formula as reported previously (25). All reactions were carried out in triplicate.
3.10. Histological Study
After blood collection and the surgical operation of rats under general anesthesia with ether, a piece of liver tissue was dissected and rapidly fixed in a 10% neutral formalin solution, then prepared by the routine procedure. Following the preparation of tissue samples in paraffin, sections of paraffin-embedded tissues with a thickness of 5 μm were cut and prepared for analysis by staining with hematoxylin-eosin (H&E) reagent (26). All sections were evaluated histologically and photographed using a light microscope.
3.11. Statistical Analysis
The statistical software SPSS (version 25, Chicago, IL, USA) was used for all analyses. The Shapiro-Wilk test was employed to check the normal distribution of variables. Since our data followed a normal distribution and the experimental groups consisted of more than three groups, we used one-way analysis of variance (ANOVA) followed by the Scheffe post hoc test to assess whether differences in parameters existed among the groups. Additionally, the repeated measures test was employed for the comparison of the time course of alterations in blood glucose level and body weight among all groups. Data were presented as mean ± SD. The figures were created using GraphPad Prism software version 6 (GraphPad, La Jolla, USA). A P-value < 0.05 was considered statistically significant.
4. Results
4.1. The Impact of CE on Blood Glucose Levels in Experimental Rats
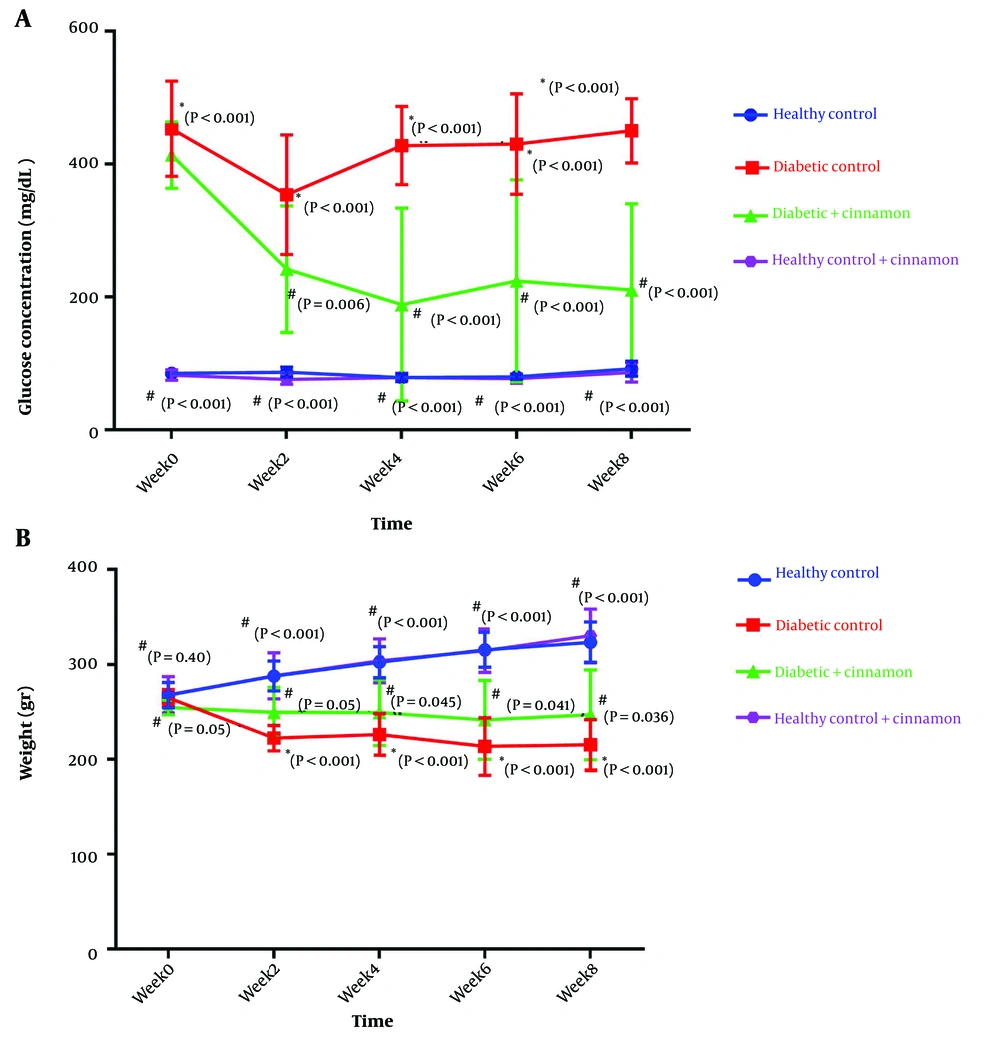
The effect of CE on glucose levels during the experiment and at the eighth week is shown in Figure 1A and Table 2, respectively. The FBG levels in rats were measured every two weeks. As expected, the glucose level in the diabetic control group was higher than that in the healthy control group throughout the experiment (Figure 1 P < 0.001). Treatment of diabetic rats with CE significantly decreased glucose levels from the second week (P = 0.006). Treatment of the healthy control rats with CE did not alter blood glucose levels in comparison to the healthy control group.
The effect of CE on FBG levels in experimental rats (A); and the effect of CE on body weight in experimental rats (B). Data indicate the mean value of the results of all animals within each group. The symbol “*” denotes the significant difference from the healthy control group and “#” denotes the significant difference from the diabetic control group.
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Healthy Control | Diabetic Control | Diabetic + Cinnamon | Healthy Control + Cinnamon | |
| FBG (mg/dL) | 91.90 ± 10.90 | 449.0 ± 48.40 < | 210.0 ± 29.90 | 86.20 ± 14.30 |
| P1 value c | ̶ | 0.001 | 0.007 | 0.80 |
| P2 value d | < 0.001 | ̶ | < 0.001 | < 0.001 |
| GSH (nmol/100 mg tissue) | 4.98 ± 0.20 | 4.75 ± 0.42 | 4.71 ± 0.25 | 4.92 ± 0.22 |
| P1 value c | ̶ | 0.07 | 0.05 | 0.60 |
| P2 value d | 0.07 | ̶ | 0.79 | 0.19 |
| MDA (nmol/100 mg tissue) | 2.51 ± 0.21 | 3.05 ± 0.47 | 2.01 ± 0.35 | 2.10 ± 0.09 |
| P1 value c | ̶ | 0.009 | < 0.001 | 0.001 |
| P2 value d | 0.009 | - | < 0.001 | 0.002 |
aThe levels of FBG and oxidative stress parameters measured after 8 weeks. Each experiment was performed duplicate.
bValues are expressed as mean (SD).
c P1 value; comparison to healthy control.
d P2 value; comparison to diabetic control.
As seen in Table 2, after eight weeks, FBG levels in the diabetic control group were considerably higher than those in the healthy control rats (449.0 ± 48.40 vs. 91.90 ± 10.90; P < 0.001). Administration of CE significantly diminished the blood glucose levels in diabetic rats (P < 0.001). The values significantly decreased from 449.0 ± 48.40 to 210 ± 29.9 mg/dL after eight weeks of treatment. Treatment of the healthy rats with CE did not have a significant effect on the FBG of these rats compared to the healthy control rats (86.20 ± 14.30 vs. 91.90 ± 10.90; P = 0.99).
4.2. The Impact of CE on Body Weight in Experimental Rats
Figure 1B shows the mean values of rats’ body weight in the different groups during the experiment. At the beginning of the study, there was no statistically significant difference in body weight among the four groups (P = 0.99). The body weight of rats in the diabetic control group exhibited a substantial drop starting from the second week compared to the healthy control group (P < 0.001). By the end of the study, the body weight of diabetic rats that received CE was significantly higher than that of the untreated diabetic rats (P = 0.036). Additionally, the body weight of healthy rats that received CE was significantly higher than that of the diabetic rats at the end of the experiment (330.4 ± 27.9 vs. 215.3 ± 26.6; P < 0.001). The animals in the healthy groups (groups I and IV) demonstrated a normal increase in body weight over time.
4.3. The Impact of CE on Oxidative Stress Parameters in Experimental Rats
As seen in Table 2, the MDA level in diabetic control rats increased significantly compared to healthy control rats (3.05 ± 0.47 vs. 2.51 ± 0.21; P = 0.009). CE feeding for eight weeks in diabetic rats resulted in significant decreases in liver MDA levels compared to diabetic controls (2.01 ± 0.35 vs. 3.05 ± 0.47; P < 0.001).
In addition, the GSH level in diabetic control rats showed no significant difference compared to healthy control rats (4.75 ± 0.42 vs. 4.98 ± 0.20; P = 0.07), and treatment of diabetic rats with CE did not result in any significant change in these animals (P = 0.79).
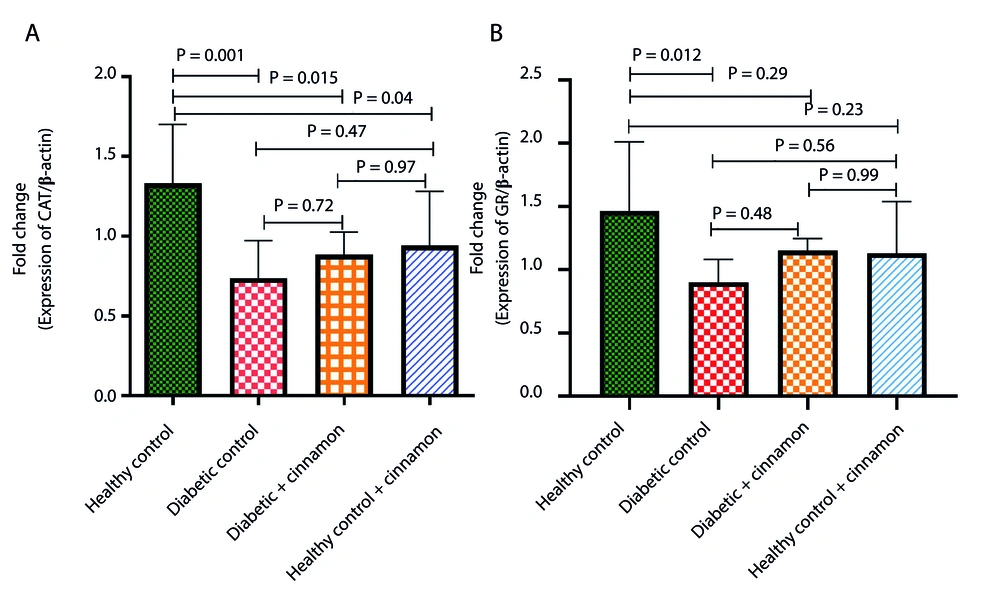
4.4. Effect of CE on GR and CAT Gene Expression in Experimental Rats
To investigate the molecular mechanism of the effects of CE on the liver, the relative mRNA expression of GR and CAT enzymes in all four experimental groups was assessed by RT-qPCR (Figure 2). The mRNA expression levels of GR (0.9 ± 0.18 vs. 1.46 ± 0.54; P = 0.012) and CAT (0.73 ± 0.23 vs. 1.33 ± 0.37; P = 0.001) were significantly decreased in the liver of diabetic rats compared to healthy control rats. Treatment of diabetic rats with CE slightly enhanced GR (0.9 ± 0.18 to 1.15 ± 0.09; P = 0.48) and CAT (0.73 ± 0.23 to 0.88 ± 0.14; P = 0.72) mRNA expression levels compared to diabetic control rats; however, these changes were not statistically significant.
4.5. The Results of Histopathology
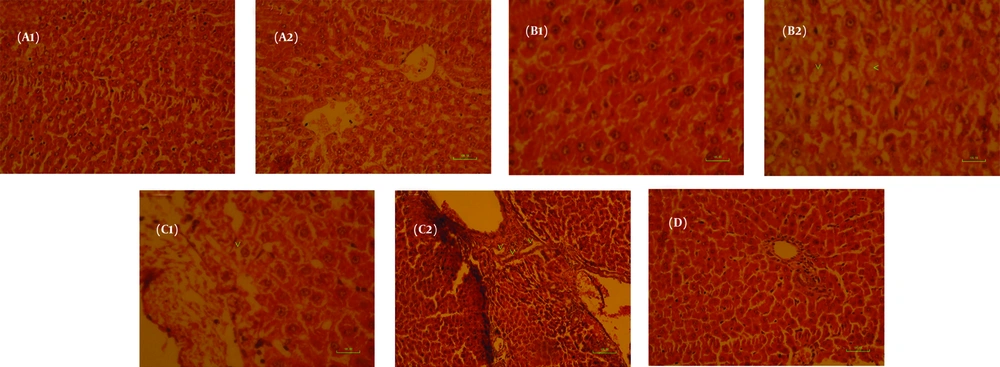
The liver tissue of the healthy control group showed normal hepatocytes and structure under light microscopic examination (Figure 3 A1 , A2). In contrast, the liver tissue of the diabetic control group exhibited mild hypertrophy in hepatocytes (arrowhead, Figure 3B) and mild hydropic degeneration. Notably, administration of CE to diabetic rats did not yield favorable outcomes; mild hypertrophic degeneration and mild biliary hyperplasia were observed in almost all hepatocytes (arrowhead, Figure C1 and C2). The liver tissue of the healthy animals that received CE showed a normal microscopic structure (Figure 3 and Table 3).
Representative histopathological profiles on the liver of healthy control rats (A1, A2); diabetic control rats (B1, B2); diabetic + Cinnamon treated rats (C1, C2); healthy control + Cinnamon treated rats (D). Scale bar is 100 μm (hematoxylin and eosin staining; magnification of A1, A2, and D are ×200; magnification of B1, B2, and C1 are ×400; magnification of C2 is ×100).
a - : Absent.
b+: Mild.
c ++ : Moderate.
5. Discussion
The main purpose of this study was to evaluate the effects of CE on antioxidant enzyme gene expression, histopathology, and antioxidant parameters in the liver of diabetic rats. Reactive oxygen species (ROS), as by-products of aerobic metabolism, are continually produced and have numerous physiological functions as well as harmful effects. Under physiological conditions, ROS are detoxified by the antioxidant defense system, which consists of antioxidant substances such as GSH, as well as antioxidant enzymes like superoxide dismutase (SOD), glutathione peroxidase (GPx), GR, and CAT.
An imbalance between oxidant and antioxidant factors and the overproduction of ROS lead to oxidative stress, which can react with and modify cellular proteins, lipids, and DNA. Oxidative stress is related to the progression of diabetes complications (27, 28). Because the liver is the most important organ involved in oxidative and detoxification processes, oxidative stress biomarkers were evaluated in the liver in this study. Additionally, experimental data show that the liver is subject to damage mediated by ROS in diabetes. Diminished body weight, polyphagia, and polydipsia are common disorders observed in diabetes (29). In this study, the induction of diabetes by STZ decreased the body weight of the rats starting from the second week compared to the rats in the healthy control group (P < 0.001). When compared to diabetic control rats, the body weight of the diabetic rats increased after receiving CE. Similar results were reported by some researchers (29-31). However, Ranasinghe et al. (32) showed a decrease in body weight in diabetic rats, and CE treatment could not increase body weight. The experiment period, dosage, and CE preparation method could account for the discrepancy between our data and the results of Ranasinghe et al. The body weight reduction in diabetic rats is likely due to the catabolism of fats and protein, as insulin deficiency results in an elevation in proteolysis of protein content in muscular tissue (33).
As demonstrated in the present study, Cinnamon was an effective agent for reducing glucose levels in serum. CE administration for diabetic rats decreased the plasma glucose level by 31% from the second week and by 53% by the end of the experiment. Our results align with those of Mahmood et al., who observed an increase in blood glucose levels in diabetic rats and a subsequent reduction after CE administration (34). Similarly, Al-Qulaly et al., Nurinda et al., Celik et al., and Boudiaf et al. showed that gavaging diabetic rats with CE significantly reduced blood glucose levels, which could be attributed to pancreatic β-cells regeneration and an elevation in insulin secretion in diabetic rats (21, 35-37). Additionally, some researchers reported that Cinnamon contains various components such as phenolic acids, steroids, flavonoids, and terpenoids that have anti-diabetic effects (38, 39). Prolonged hyperglycemia due to diabetes causes an increase in the production of free radicals, which alter cellular functions and induce lipid peroxidation. The lipid peroxides attack cell membranes, damaging proteins and DNA, leading to various diabetic complications. In the present study, the liver MDA level, a marker of lipid peroxidation, increased in diabetic rats, while administration of CE significantly diminished the MDA level close to the normal value (40). Our results align with previous studies that reported Cinnamon reduced MDA concentrations in the serum, liver, and kidney of diabetic rats (15, 16, 29). GSH is known as a critical free radical scavenger in the intracellular region, mainly maintaining the antioxidant status in plasma and cells (40). In this study, the GSH level in the liver of rats was determined, and the results showed that this parameter was not significantly altered in all groups. Some studies have demonstrated that the induction of diabetes in rats results in the diminishing of GSH levels, and administration of CE for these rats significantly increased GSH levels. The discrepancy between our results and the results of Mohammed et al. is probably due to various factors, such as different study models, CE dose, Cinnamon preparation, rat species, and so forth. We used a water extract of Cinnamon at a dose of 40 mg/kg, B.W. in Sprague Dawley rats, but Mohammed et al. treated albino Wistar rats with nano-encapsulated Cinnamon oil at doses of 200 mg/kg and 400 mg/kg (15).
According to our results, the mRNA expression of the antioxidant enzymes CAT and GR in diabetic rats significantly decreased compared to the healthy control group. While administration of CE for diabetic rats could not significantly increase the mRNA expression of CAT and GR. Niazmand et al. reported that the activity of CAT in the liver homogenate of diabetic rats decreased and CE could increase this enzyme’s activity. In their study, the expression of the CAT gene was not investigated (41). The difference between our results and the previous study is due to the study design. In general, CAT decomposes hydrogen peroxide (H2O2) derived from SOD activity into water and oxygen. In animals, H2O2 is detoxified by CAT and GPx, and H2O2 regulates CAT gene expression. Because catalase shares its substrate with glutathione peroxidase, no significant change in the expression of the catalase gene after CE administration may be due to H2O2 being involved in the reaction of glutathione peroxidase (42). The GR enzyme converts oxidized glutathione to its reduced form (GSH) by oxidizing NADPH. Some studies have reported that the activities of CAT, GPx, and SOD in the liver of diabetic rats are diminished compared to control rats, and cinnamon can reverse these effects after 4 or 6 weeks (40, 43). Our results align with these studies regarding the reduction of antioxidant enzyme activity following diabetes induction. However, the administration of CE couldn’t restore these enzymes at the gene expression level. Conversely, other researchers have demonstrated that the activity of SOD, CAT, and GR enzymes increased in diabetic rats after 5 - 6 weeks (44, 45). However, none of these researchers studied these enzymes at the gene expression level. It has been shown that oxidative stress in a cell can stimulate the transmission of transcription factors that are sensitive to redox conditions into the nucleus. Therefore, changes in the gene expression of antioxidant enzymes in diabetes are likely due to the oxidation of transcription factors, which affects the initiation of transcription of these enzyme genes (46). Liver histology in the healthy control group demonstrated normal structure and cellular construction, including the portal triad, numerous hepatocytes, and the central vein. The histopathology of the liver in STZ-diabetic animals showed mild hypertrophy in hepatocytes, consistent with the results of previous studies (15, 30). Interestingly, the administration of CE (40 mg/kg B.W) had no protective effects on hepatocyte structure in diabetic rats. The hypertrophy of hepatocytes may be due to an increase in intracytoplasmic eosinophilic granules in hepatic cells and an elevated level of general oxidative stress in diabetic conditions (15). Mohammed et al. reported that the administration of nano-encapsulated cinnamon oil (400 mg/kg B.W) improved the structure of hepatic cells in diabetic rats. The discrepancy between our results and those of Mohammed et al. is likely due to differences in CE dose, cinnamon extract preparation, and rat species (15).
In conclusion, the findings of our study indicate that the administration of CE at 40 mg/kg for STZ-induced diabetic rats significantly normalized hyperglycemia and lipid peroxidation after 8 weeks. However, this herbal medicine could not normalize the histopathological changes that occurred in diabetic rats. Additionally, since this extract has adverse effects on hepatocytes, its prescription as an anti-diabetic agent should be done with caution and should include liver examination. Although this study showed that cinnamon had a significant positive impact on the antioxidant system in diabetes, further well-planned randomized clinical trials are required to confirm this regimen as a safe and useful remedy. Additionally, more research should be conducted to clarify the other molecular processes contributing to cinnamon's protective effects against diabetes, beyond its impact on the human liver.