1. Background
Acute myocardial infarction (AMI) is a critical coronary event that carries a high risk of morbidity and mortality, representing the most severe manifestation of coronary artery disease (1). Given the prevalence of ST-segment elevation myocardial infarction (STEMI), accurately assessing the severity of the condition and predicting patient recovery is crucial. Historically, a variety of clinical and echocardiographic indicators have been explored to identify predictors of ventricular recovery (2, 3).
The introduction of speckle-tracking echocardiography (STE) has transformed echocardiography from a largely subjective assessment into an objective analysis with diagnostic parameters (4). In recent years, the global longitudinal strain (GLS) metric has emerged as a more reliable and sensitive measure for evaluating left ventricular (LV) function (5). Research has shown that GLS can serve as a prognostic indicator in a broad range of patients, including those with cardiovascular and valvular diseases, covering conditions like chronic heart failure and aortic stenosis (6, 7). GLS is particularly adept at identifying declines in LV function, even when left ventricular ejection fraction (LVEF) remains within normal ranges (8). Previous studies indicate that GLS offers superior predictive value in estimating infarct size (IS) shortly after reperfusion therapy in patients with STEMI (9). Additionally, GLS has proven more accurate than traditional echocardiography methods in determining IS during subsequent follow-ups (10). Consequently, GLS has the potential to be a valuable indicator of ventricular damage.
Identifying accurate prognostic markers allows for the assessment of how various baseline variables might influence the likelihood of recovery after an AMI. The type of revascularization (primary versus rescue versus deferred percutaneous coronary intervention (PCI)) could be one such variable and a key factor in determining ventricular recovery. Currently, there is a limited amount of research examining the relationship between the mode of revascularization and recovery outcomes, although studies have been carried out to assess the early and in-hospital outcomes of each revascularization strategy, including mortality and major adverse cardiovascular events (MACEs) (11).
2. Objectives
In this study, our objective was to investigate the link between ventricular function improvement post-STEMI and GLS and to determine the predictive power of baseline GLS for LV function recovery after STEMI. Additionally, we aimed to determine whether the type of primary revascularization differed among patients who did and did not experience recovery during the follow-up period.
3. Methods
3.1. Study Design and Participants
In this cohort study, patients who presented with STEMI and were admitted to Al-Zahra Hospital, a tertiary referral center, for PCI were prospectively enrolled from March 2022 to March 2023. Eligibility for inclusion was determined for all patients who underwent successful PCI on the offending lesion and had an estimated LVEF of less than 40% within 48 hours post-PCI, as evaluated by echocardiography. Exclusion criteria included: (1) A prior diagnosis of AMI, (2) any form of previous revascularization such as PCI or coronary artery bypass grafting (CABG), (3) significant valvular heart disease, and (4) the occurrence of any MACEs during the 6-month follow-up period, including mortality, early rehospitalization, or significant arrhythmia. Participants were divided into two groups based on the improvement in LV systolic function. Improvement in LV systolic function was defined as an increase in LVEF of ≥ 5% as assessed by echocardiography 6 months after the STEMI event. Upon the enrollment of eligible patients, their baseline demographic information, comorbidities, treatment data, and echocardiographic parameters were recorded. All participants provided signed written informed consent before joining the study, which had received approval from the university's institutional review board. The study was conducted in accordance with the principles set forth in the Declaration of Helsinki.
3.2. Sample Size Determination
For the objectives of this study and based on the outcomes of a previous related study, we determined the necessary sample size with a 5% Type I error and 80% power. A prior study demonstrated an area under the curve (AUC) of 0.73 for the predictive value of GLS in the recovery of left ventricular function (12). The ratio of negative to positive groups in their sample was 16 to 14. Assuming a null hypothesis value of 0.5, we calculated the sample sizes for the groups without recovery and with recovery to be 26 and 22, respectively, using MedCalc Version 22.005.
3.3. Echocardiographic Assessment
Standard transthoracic echocardiography (TTE) was conducted at baseline and after 6 months of follow-up for all patients. A cardiologist, who was not informed of the angiography findings, performed all echocardiographic evaluations. The echocardiography was carried out with patients in the left lateral decubitus position. Using GE Vivid 9 equipment, conventional echocardiography and color Doppler imaging were obtained. The LVEF was measured employing the biplane Simpson's rule. Measurements of the left ventricular dimensions at end-systole (left-ventricular end-systolic volume (LVESV)) and end-diastole (left-ventricular end-diastolic volume (LVEDV)) followed the American Society of Echocardiography's recommendations (13). The GLS measurement utilized STE on two-dimensional grayscale images. Left ventricular longitudinal strain analysis was conducted using the standard 17-segment model, with standard views of the four-chamber, two-chamber, and long-axis. Each view included six myocardial segments, with each strain curve representing the average strain value of an individual myocardial segment. GLS was calculated as the average of all peak systolic strain values. Myocardial contractile recovery was defined as an improvement of ≥ 5% in LVEF on follow-up echocardiography.
3.4. Outcomes
As previously mentioned, patients were divided into two categories, recovery and non-recovery, based on the status of their left ventricular (LV) systolic function recovery as determined by TTE during follow-up evaluations. The baseline, final, and absolute changes in echocardiographic parameters, including LVEF, LVEDV, LVESV, and GLS, were compared between the recovery and non-recovery groups. Furthermore, the types of reperfusion therapy (primary PCI, rescue PCI, and deferred PCI after 24 hours) were analyzed by the two groups. Changes in echocardiographic markers from baseline were also examined between groups according to their revascularization type.
3.5. Statistical Analysis
Categorical variables were displayed as frequency (percentage), while continuous variables were presented as mean (standard deviation (SD)). The paired-sample t-test was employed to assess significant differences within groups. The independent-sample t-test was utilized to compare the means of baseline and final echocardiographic parameters between the groups, and a mean difference (MD) along with the associated 95% confidence interval (CI) were provided. Differences among groups based on their revascularization type were evaluated using the analysis of variance (ANOVA) test. For categorical variables, the chi-square test was applied to compare outcome measures. A receiver operating characteristic (ROC) curve analysis was performed to identify an optimal cut-off value for baseline GLS in predicting the recovery status of LV systolic function during the follow-up assessment. The ROC curve analysis included an estimate of the effect size, reported as the AUC with 95% CI, and the related P-value. AUC values were interpreted as follows: 0.5 indicated no discrimination, 0.5 - 0.7 indicated poor discrimination, 0.7 - 0.8 indicated acceptable discrimination, and > 0.8 indicated excellent discrimination (14). A two-tailed P-value of < 0.05 was considered statistically significant for all analyses. MedCalc Software version 19 was utilized for the ROC curve analysis, while SPSS version 26.0 (SPSS Inc., Chicago, IL, USA) was used for all other statistical analyses.
4. Results
4.1. Baseline Characteristics
A total of 60 consecutive patients diagnosed with STEMI underwent both baseline and 6-month follow-up echocardiography and were subsequently included in the analysis. The average age of the entire cohort was 56.57 ± 7.48 years, with males comprising 73.3% of the participants. By the 6-month follow-up, 38 (63.3%) patients had experienced a recovery, defined as a ≥ 5% increase in LVEF. All participants received optimal treatment aligned with the most recent STEMI management guidelines. Among the baseline comorbidities, hypertension was notably more common in the group that did not show recovery at 6 months (P-value < 0.001). Table 1 provides a detailed overview of the baseline characteristics and laboratory findings for each group.
| Demographic Determinants | Groups | P-Value | |
|---|---|---|---|
| Recovered (n = 38) | Not recovered (n = 22) | ||
| Age | 54.52 ± 7.39 | 60.00 ± 7.63 | 0.008 |
| Gender (male) | 30 (78) | 14 (77) | 0.99 |
| Diabetes mellitus | 8 (21) | 4 (18) | 0.99 |
| Hypertension | 4 (10.5) | 12 (54.5) | < 0.001 |
| Hyperlipidemia | 8 (21) | 4 (18.2) | 0.99 |
| Smoking | 16 (42.1) | 12 (54.5) | 0.425 |
| WBC | 9.71 ± 2.00 | 8.46 ± 3.13 | 0.104 |
| Hemoglobin, g/dL | 13.98 ± 1.70 | 12.96 ± 1.25 | 0.018 |
| Platelets | 220.68 ± 47.45 | 232.09 ± 124.21 | 0.683 |
| BUN | 15.89 ± 3.63 | 19.82 ± 5.88 | 0.008 |
| Creatinine | 0.94 ± 0.18 | 0.97 ± 0.21 | 0.42 |
| Na | 139.84 ± 4.04 | 138.45 ± 2.52 | 0.152 |
| K | 4.23 ± 0.37 | 3.82 ± 0.25 | < 0.001 |
| SBP, mmHg | 128.95 ± 12.42 | 131.82 ± 28.05 | 0.653 |
| DBP, mmHg | 81.05 ± 11.52 | 82.73 ± 13.16 | 0.608 |
Baseline Characteristics a
4.2. Echocardiographic Parameters
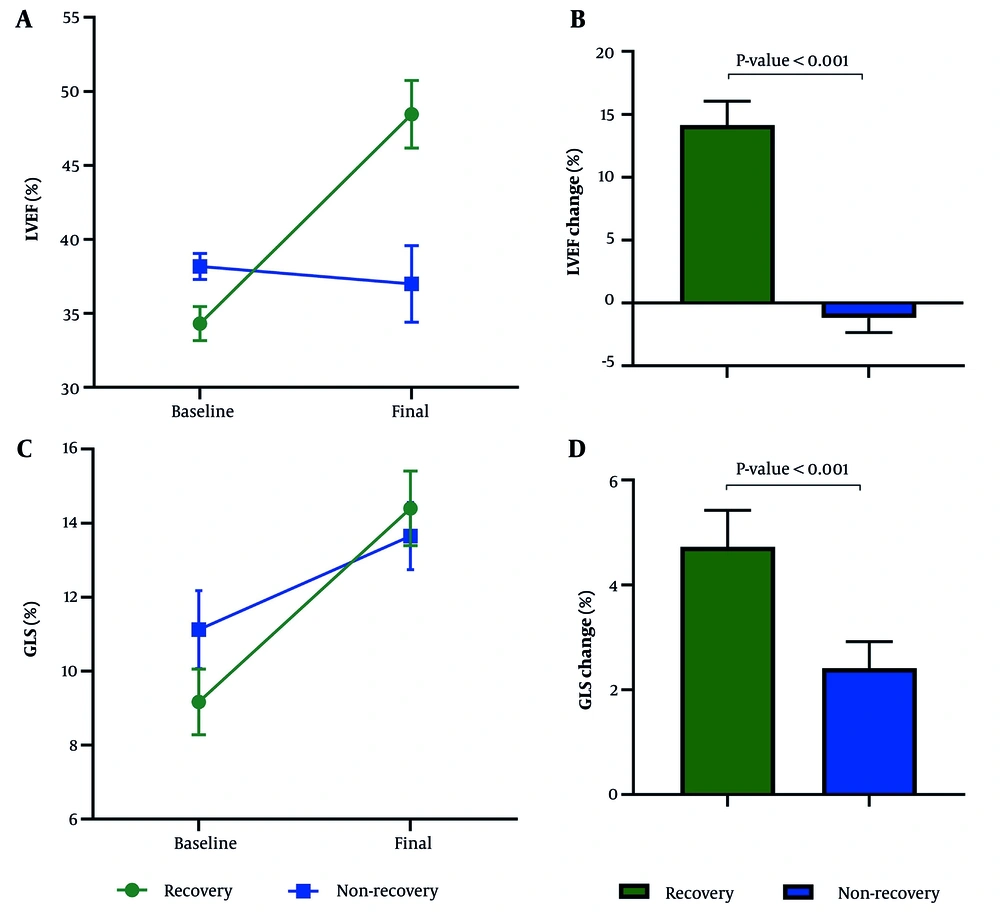
The initial LVEF measured by TTE averaged 34.32 ± 3.51 in the recovery group and 38.18 ± 1.99 in the non-recovery group, showing a significant difference (P-value < 0.001). The mean change in LVEF was 14.16 ± 5.75 in patients who recovered, in contrast to a decrease of -1.18 ± 5.41 in those who did not recover (MD (95% CI) = 15.34 (12.32; 18.36), P-value < 0.001). The average baseline GLS improved from -9.17 ± 2.55 to -14.40 ± 3.07 in the recovery group, which saw an improvement in LVEF of more than 5% after six months, while it altered from -11.12 ± 2.15 to - 13.66 ± 2.05 in the group that did not recover (P-value = 0.008). The change in GLS averaged -4.73 ± 2.01 in recovered patients and - 2.41 ± 2.16 in the non-recovery group (MD (95% CI) = -2.31 (-1.11; -3.52), P-value < 0.001) (Figure 1). The initial LVESV was 65.57 ± 12.15 in the recovery group and 58.62 ± 17.57 in the non-recovery group (P-value = 0.169). The change in LVESV was significantly less in patients with LVEF improvement compared to those without recovery (recovery: - 9.69 ± 15.87 vs. non-recovery: 2.34 ± 14.95 (MD (95% CI) = - 12.03 (- 21.63; - 2.44), P-value = 0.015)). The average initial and final left-ventricular end-diastolic volume (LVEDV) was 100 ± 20.93 and 102.58 ± 27.47 in recovered patients, whereas it was 94.25 ± 27.78 and 99.18 ± 15.69 in the non-recovery group (MD of between-group change = - 2.38 (- 13.91; 9.16), P-value = 0.68). Table 2 showcases the comparison of echocardiographic parameters.
| Variables | Groups | P-Value | |
|---|---|---|---|
| Recovered (n = 38) | Not recovered (n = 22) | ||
| LVEF, % | < 0.001 | ||
| Baseline | 34.31 ± 3.51 | 38.18 ± 1.99 | < 0.001 |
| Six months | 48.47 ± 6.95 | 37.00 ± 5.83 | < 0.001 |
| Change | 14.15 ± 5.75 | -1.18 ± 5.41 | |
| LVEDD, mm | 0.73 | ||
| Baseline | 50.70 ± 6.70 | 51.33 ± 4.92 | 0.10 |
| Six months | 55.00 ± 5.42 | 57.18 ± 4.53 | 0.71 |
| Change | 5.23 ± 8.57 | 5.88 ± 4.10 | |
| LVESD, mm | 0.01 | ||
| Baseline | 38.35 ± 7.08 | 34.22 ± 4.25 | 0.12 |
| Six months | 43.78 ± 8.39 | 40.36 ± 7.70 | 0.97 |
| Change | 6.58 ± 8.82 | 6.66 ± 5.71 | |
| LVEDV, mL | 0.47 | ||
| Baseline | 100.00 ± 20.92 | 94.25 ± 27.78 | 0.54 |
| Six months | 102.58 ± 27.47 | 99.18 ± 15.69 | 0.68 |
| Change | 5.37 ± 23.22 | 3.00 ± 15.89 | |
| LVESV, mL | 0.17 | ||
| Baseline | 65.56 ± 12.15 | 58.61 ± 17.56 | 0.26 |
| Six months | 53.54 ± 18.24 | 62.42 ± 10.57 | 0.02 |
| Change | -9.69 ± 15.86 | 2.33 ± 14.94 | |
| GLS, % | 0.01 | ||
| Baseline | -9.17 ± 2.55 | -11.12 ± 2.14 | < 0.001 |
| Six months | -14.40 ± 3.07 | -13.65 ± 2.04 | < 0.001 |
| Change | -4.72 ± 2.00 | -2.41 ± 2.15 | |
Echocardiographic Indices Compared Between Recovered and Not Recovered Participants
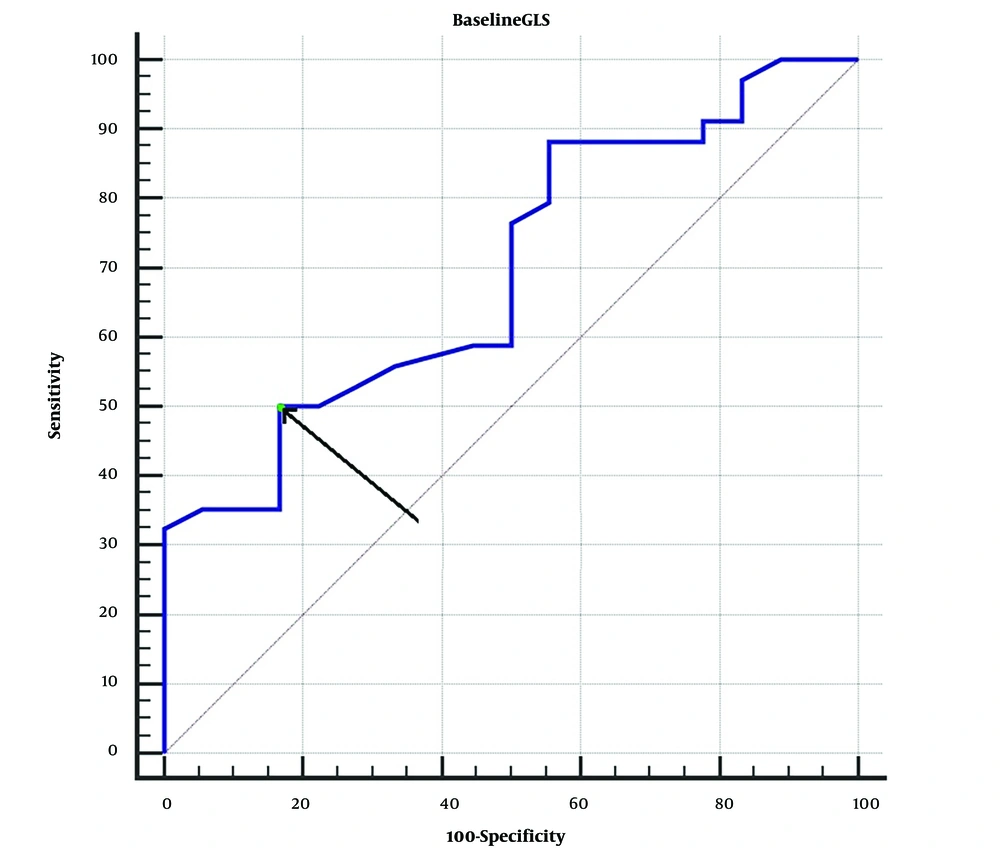
4.3. Receiver Operating Characteristic Curve Analysis of LV Recovery Based on Baseline GLS
Based on the ROC curve analysis, a baseline GLS of ≤ -4.5 and > -14.8 was able to accurately predict unsuccessful or successful recovery of LVEF with 100% negative and positive predictive values, respectively. Baseline GLS values greater than -9.2 were predictive of LV recovery with a sensitivity of 50% (range 37.6 - 62.4%) and a specificity of 83.3% (range 67.2 - 93.6%) (P = 0.0002, AUC (95% confidence interval) = 0.697 (0.594, 0.799)) (Figure 2).
4.4. Treatment Modalities
Patients were categorized based on the type of revascularization they received at baseline. Of the total, 36 patients underwent primary PCI, 12 received rescue PCI, and 12 were treated with deferred PCI 24 hours post-STEMI diagnosis. Within the primary PCI cohort, 24 patients (66.7%) exhibited a ≥ 5% improvement in LVEF at follow-up. In contrast, the rescue PCI group saw 10 patients (83.3%) recover, while the deferred group had 4 recoveries (33.3%). There was a significant correlation between the initial type of revascularization and LVEF recovery at follow-up (P-value = 0.032), with the deferred PCI group showing a markedly lower recovery rate compared to the primary and rescue PCI groups (P-values = 0.043 and 0.013, respectively). Although the recovery rate was higher in the rescue group than in the primary group (83.3% vs. 66.7%), this difference was not statistically significant (P-value = 0.271) (Table 3).
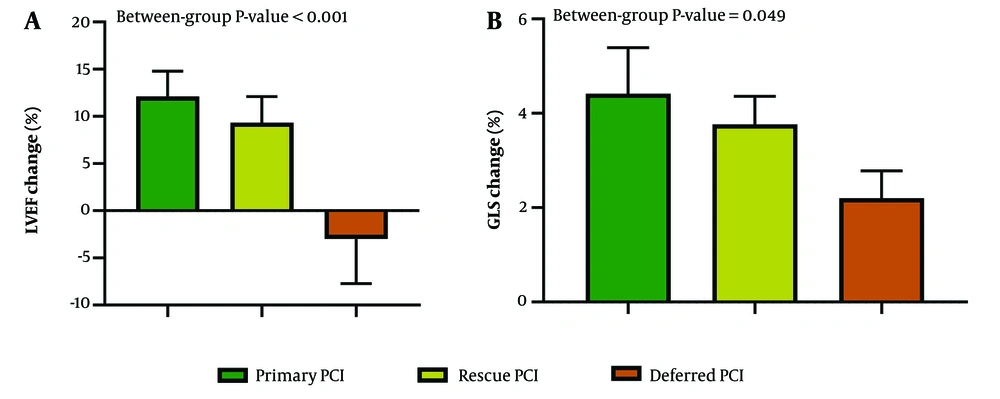
The study also assessed the relationship between changes in echocardiographic parameters and the initial mode of revascularization. Changes in all measured echocardiographic parameters (LVEF, LVEDV, LVESV, and GLS) were significantly associated with the type of revascularization performed (Table 4 and Figure 3).
| Variables | Type of Revascularization | P-Value | ||
|---|---|---|---|---|
| Primary PCI | Rescue PCI | Deferred PCI | ||
| LVEF change, % | 12.11 ± 7.99 | 9.33 ± 4.37 | -3.00 ± 7.43 | < 0.001 |
| LVEDV change, mL | -5.37 ± 16.06 | 17.50 ± 17.63 | 31.50 ± 3.74 | < 0.001 |
| LVESV change, mL | -12.46 ± 13.07 | -4.76 ± 5.01 | 20.49 ± 6.19 | < 0.001 |
| GLS change, % | -4.41 ± 2.72 | -3.76 ± 0.94 | -2.2 ± 0.75 | 0.049 |
Change in Echocardiographic Indices Stratified by the Type of Revascularization a
Comparison of LVEF and GLS change from baseline to 6-month follow-up compared between groups based on their initial type of reperfusion therapy. Error bars show 95% CI. LVEF: Left ventricular ejection fraction; GLS: Global longitudinal strain; PCI: Percutaneous coronary intervention; CI: Confidence interval.
5. Discussion
In this study, our objective was to investigate the prognostic value of baseline GLS measurements in predicting the recovery of systolic function in patients with STEMI undergoing PCI. Our findings indicate that while the measurement of GLS within 48 hours post-STEMI is statistically significant, it may not robustly predict an improvement of ≥ 5% in LVEF six months post-event. Patients who experienced a ≥ 5% improvement in LVEF displayed significantly higher baseline GLS values and greater changes in GLS from baseline to the final follow-up compared to those who did not show recovery. Despite the ROC curve analysis showing baseline GLS as a significant predictor of contractile recovery (P < 0.001), the results remained inconclusive due to a broad confidence interval for the AUC (0.55, 0.82). While cut-off values of -4.5 and -14.8 could accurately forecast the failure and success of ventricular recovery with 100% negative and positive predictive values, respectively, the values in between these thresholds could not reliably distinguish between recovery and non-recovery. In a prospective study of 147 AMI patients treated with primary PCI, 48% achieved recovery within a year, and an initial GLS cut-off value of -13.7 offered 86% sensitivity and 74% specificity in predicting long-term recovery (AUC: 0.82 to 0.93) (15).
In another investigation by Shehata et al., significant changes in GLS were observed in both groups of patients receiving PCI and those treated with thrombolytics. Although specific criteria for myocardial recovery were not explicitly defined, multivariate regression analysis revealed that baseline GLS was a strong predictor of myocardial function recovery, alongside myocardial performance index and systolic myocardial excursion, but not baseline LVEF. Notably, the change in GLS values from baseline to the 3-month follow-up positively correlated with ST-segment resolution and negatively with the duration from door to perfusion time (16).
The potential role of strain imaging in predicting an improvement of ≥ 5% in LVEF was examined in a single-center study involving 100 AMI patients undergoing PCI. The study determined that the optimal cut-off value for baseline GLS predicted recovery with relatively low sensitivity (53%) and a broad 95% confidence interval for the AUC (0.72 (0.55 - 0.87)), suggesting the possibility of inconclusive outcomes (12). Given the current evidence, employing baseline GLS as an indicator of ventricular function recovery should be approached with caution due to mixed findings regarding its significance across different studies. Further investigation of myocardial strain imaging as a recovery predictor is recommended in future studies with propensity matching to reduce the influence of potential confounders inherent in observational studies.
Identifying reliable predictors of left ventricular function recovery at the initial presentation in patients with STEMI is crucial. The prognostic value of various potential recovery predictors has been explored, including initial LVEF, IS, usage of statins, the territory of AMI, beta-blocker usage, and the absence of diabetes as previously established markers of recovery in AMI patients (2, 16-18). Regarding pre-existing comorbidities, the presence of hypertension has been strongly linked to increased mortality and reinfarction rates in the context of STEMI (19). In our study, a notably higher percentage of patients in the non-recovery group (54% vs. 10%) had hypertension as a comorbidity compared to those who recovered, highlighting the significance of effective long-term blood pressure management in preventing adverse clinical outcomes following AMI (20).
We further categorized patients based on their initial type of PCI and assessed the potential link between the type of revascularization received at baseline and subsequent improvement in LVEF. Previous research has indicated that for patients with AMI, there might be differences in angiographic characteristics, such as the culprit coronary artery, between those undergoing primary and rescue PCI. However, in-hospital outcomes, including mortality and MACEs, did not show significant differences between these revascularization strategies (11). In alignment with prior research, our findings indicate that both primary and rescue PCI is associated with higher recovery rates compared to deferred PCI performed after 24 hours. Additionally, patients treated with primary and rescue PCI exhibited improvements in LVEF of 12.1% and 9.3%, respectively, at the 6-month follow-up, whereas those who underwent deferred PCI experienced a 3% reduction in their LVEF values. These results underscore the conclusion that any revascularization delays in STEMI patients are closely linked to poorer clinical outcomes and potentially lasting left ventricular dysfunction.
The relatively modest sample size of this study stands as one of its limitations, making our results potentially less applicable to the broader STEMI patient population. The influence of confounding factors on the outcomes of any observational study cannot be overlooked, and our investigation is no exception to this rule. Additionally, the 6-month period for evaluating ventricular function recovery might not capture patients who exhibit delayed improvement. We did not conduct follow-up angiography to assess the incidence of restenosis and re-occlusion, nor did we report on long-term MACEs in our study. Furthermore, we lacked data on several additional characteristics and parameters, such as the identity of the culprit coronary artery, information on wall motion abnormalities, and regional longitudinal strain measurements.
5.1. Conclusions
In conclusion, while baseline strain imaging might serve as a potential predictor of recovery in patients with AMI, caution is warranted in its application since our findings did not establish a robust link between initial GLS and subsequent improvement in LVEF. Although baseline GLS values of ≤ -4.5 and > -14.8 were predictive of either failure or success in ventricular recovery with 100% negative and positive predictive values, respectively, the values falling between these thresholds did not consistently distinguish between recovery and non-recovery outcomes. On the other hand, the type of revascularization may serve as an indicator of LVEF recovery, with patients undergoing primary and rescue PCI likely to experience better outcomes and more significant improvements in LVEF compared to patients receiving delayed PCI after 24 hours.