1. Introduction
Tuberculosis (TB) is a chronic granulomatous disease ranking second leading cause of death by infectious agents in 2021. It is estimated that 10.6 million got infected and 1.6 million died of TB around the world, making it a major health problem (1). Although extrapulmonary tuberculosis (EPTB) accounts for a small proportion of all TB cases, valvular TB is a particularly rare manifestation of the disease, with an estimated incidence of less than 1% of EPTB cases (2). Valvular TB tends to occur more frequently in developing countries and among individuals with weakened immune systems, such as those living with human immunodeficiency virus. Diagnosis typically involves a combination of imaging and laboratory evaluations, including blood tests and cultures of bodily fluids. Treatment for valvular TB typically involves a prolonged course of multiple antibiotics to eradicate the infection and might require surgical intervention to repair or replace damaged heart valves (3).
2. Case Presentation
A 60-year-old female patient with a medical history of diabetes mellitus (DM) and rheumatoid arthritis (RA) was referred to a cardiology clinic for a cardiac assessment prior to a hand-wound debridement surgery. In the course of obtaining the patient’s medical history, there was no indication of any relevant clinical symptoms, including weakness, lethargy, fatigue, fever, or chills. The patient’s RA was inactive at the time of examination, and her DM was under control. The patient’s drug history included hydroxychloroquine and naproxen for RA and insulin for DM.
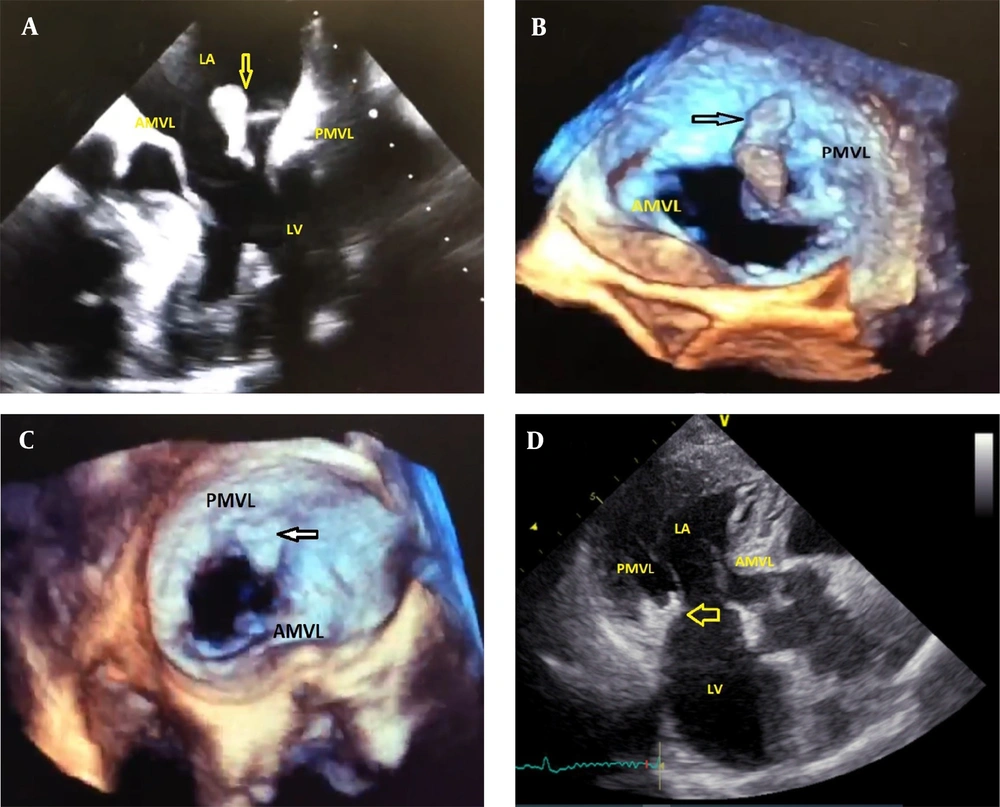
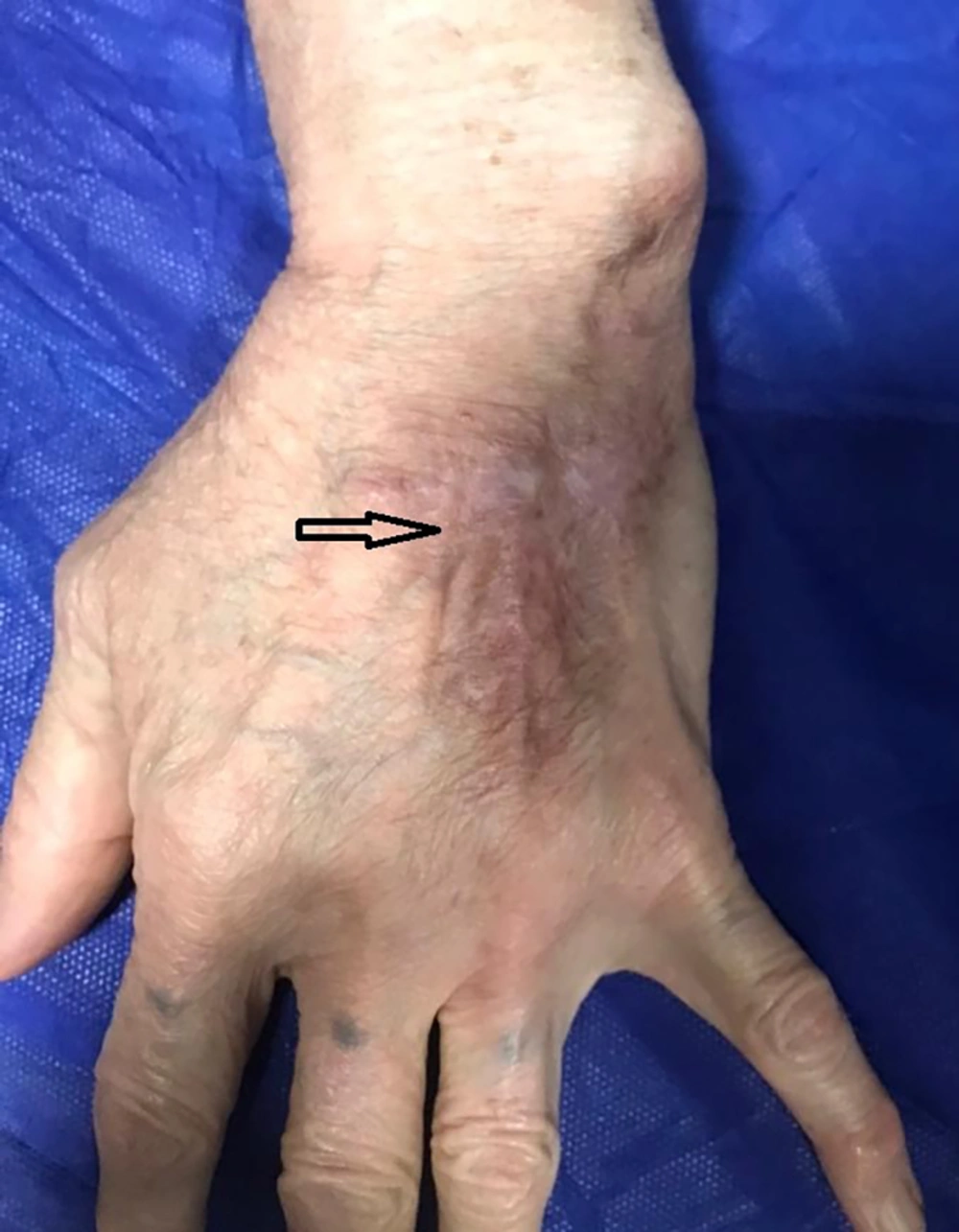
Additionally, during physical examination, a significant wound was observed on the dorsal side of the patient’s left hand. The auscultation of the patient’s heart yielded a grade II-III holosystolic murmur over the mitral area; nevertheless, lung auscultation revealed no pathological findings, and no petechiae or purpura or other lesions were observed on the patient’s skin. A 12-lead electrocardiogram was obtained, which was normal. Due to the presence of the murmur, transthoracic echocardiography (TTE) was performed. Despite the absence of symptoms of systemic embolism, including a history of cerebrovascular accident or peripheral embolism, a mass was identified on the patient’s mitral valve during TTE, along with a moderate mitral regurgitation (MR) (Figure 1A). A subsequent transesophageal echocardiography (TEE) was performed, revealing a large, highly mobile mass in the posterior leaflet of the mitral valve, an inhomogeneous abscess-like density around the ring and annulus of the valve, and a moderate MR (Figure 1B).
A, Large size highly mobile mass on TTE view; B, Large highly mobile mass on TEE view; C, Remnant mass on PMVL in repeated TEE (arrow in TEE view); D, No mass on leaflet after the treatment, arrow indicates the residual thickness in the MV ring which has been shrunken (3 chamber view of TTE). PMVL, posterior mitral valve leaflet; AMVL, anterior mitral valve leaflet; LA, left atrium; LV, left ventricle; TTE, transthoracic echocardiography; TEE, transesophageal echocardiography.
The patient’s laboratory results indicated an increase in C3 complement and rheumatoid factor (RF) levels; however, C4, CH50, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), and platelet levels were within the normal range, and her hemoglobin level was slightly low (8.4 g/dL). To assess the possibility of infective endocarditis, a blood culture study was performed, which revealed negative results on consecutive blood specimens. Due to the large size and the high mobility of the mass, cardiac surgery was planned. In preparation for the surgical intervention, a cardiac angiography was conducted to assess the patency of the coronary vessels, which revealed no obstructive lesions. However, due to the reluctance of the patient, the cardiac surgery was canceled, and a dermatological consultation was requested to investigate the origin of the wound on the patient’s hand.
A biopsy of the wound was taken upon the dermatologist’s suggestion. Despite the absence of clinical or paraclinical evidence of infective endocarditis, the patient was treated with anticoagulant (heparin) and antibiotics for 5 days. On the fifth day after the initial TEE, the patient decided to proceed with the cardiac surgery; therefore, a follow-up TTE was performed, revealing no obvious mass on the mitral valve, and no clinical or paraclinical evidence of systemic embolism was observed. A second TEE performed on the eighth day after the initial TEE showed a small remnant mass in the posterior leaflet of the mitral valve and an inhomogeneous abscess-like density in the ring and annulus of the valve (Figure 1C). Given the rapid improvement of the mass with heparin treatment, the mass was presumed to be a thrombus. Although surgical intervention was initially considered due to the thickness around the ring, it was recommended to postpone the surgery until the biopsy results were obtained due to the lack of an accurate diagnosis and the high risk associated with surgery.
Ten days after the initial TEE, the skin pathology results indicated a necrotizing granuloma, and no fungal elements or Leishman body were observed. A polymerase chain reaction (PCR) test for mycobacterium was recommended, and a subsequent PCR test performed on the wound sample was positive for Mycobacterium tuberculosis. Although a chest computed tomography (CT) scan showed no evidence of lung involvement with TB, the infectious disease specialist suggested the possibility that the abscess-like density around the ring was also caused by TB granuloma. Therefore, the patient was treated with quadruple anti-TB medication for 24 weeks, during which the wound on her hand began to heal. After completing the treatment, a follow-up TTE revealed that the abscess-like density around the ring had shrunk, and the valve mass was no longer visible, with a mild to moderate MR (Figure 1D). The patient’s cardiac and hand wound debridement surgery was ultimately canceled, and no cardiac complications were reported during the one-year follow-up period (Figure 2).
3. Discussion
Herein, we report a case of a female patient with DM who presented with a skin wound on her hand that required wound debridement surgery. During the preoperative cardiology assessment, an echocardiogram revealed a mass adjacent to the mitral valve, although the patient did not have any complaints relating to the heart.
Tuberculosis is considered to be a major public health problem, especially in low-income countries, since it is the second leading cause of death by a single infective agent (after coronavirus disease 2019 [COVID-19]) (1). Furthermore, due to the ability of TB to involve multiple organs in the body, the morbidity is high, making it a heavy burden for society. Tuberculosis mostly involves the respiratory system; however, it can also involve other organs. The extrapulmonary form is not as prevalent as the usual form, with a prevalence of 27.7% among all TB cases in Iran (4). The most commonly affected sites in EPTB are lymph nodes and pleura, and however, rarely, it can involve different heart layers, including the endocardium, with a reported prevalence of about 0.6% among TB patients in a case series study in 1906 on 7 683 cases (3).
Diagnosing cardiac TB poses numerous challenges given that most individuals infected with TB are unsuspected, without any significant symptoms, and are diagnosed postmortem (5). The direct detection of mycobacterium or its products is the gold standard for identifying TB as the cause of endocarditis (6). However, this approach can be difficult due to the non-uniform distribution of bacteria and the challenge of accessing the site. As a result, clinicians often rely on clinical history, physical examination, blood testing, and echocardiography to diagnose the condition. Echocardiography is the first-line tool to assess suspected patients, with valve vegetation and insufficiency being the most common findings. The mitral and aortic valves are the most commonly affected by vegetation (7). Similarly, a TTE revealed vegetation on our patient’s mitral valve, which was later depicted as a mobile, large-sized mass on the posterior leaflet of the mitral valve, along with an abscess-like density and a moderate MR. Moreover, due to the subsequent pathological findings of the patient’s wound, cardiac TB became our top-of-the-list differential diagnosis.
As mentioned earlier, diagnosing cardiac TB is a challenge to clinicians, and there is often reluctance among clinicians to start anti-TB chemotherapy until a definite diagnosis is made. Therefore, the delay in initiating appropriate management can sometimes put the patient’s health in jeopardy, as the patient’s hemodynamics can be altered by vegetation (3, 8). This challenge is compounded by the inability of TTE to diagnose certain TB vegetations, as they can vary in size from millimeters to a few centimeters. Although TEE appears to have a higher sensitivity in detecting native-valve infectious endocarditis compared to TTE (9), there is not much data regarding the comparison of these two methods in assessing cardiac TB.
The management of cardiac TB is based on surgical replacement of damaged valves, combined with a radical quadruple anti-TB treatment (3, 7). However, not all cases require surgical valve replacement, and there are reported cases of cardiac TB treatment using only an anti-TB regimen, with a significant reduction in the mass size after the treatment initiation (10-13). Therefore, pharmacological treatment can be considered both a diagnostic and management tool in suspected cases (10), and surgical intervention can be reserved for cases with severe valve dysfunction or inadequate response to anti-TB treatment (14, 15).
In this case, our patient showed a remarkable response to the therapeutic intervention administered, as evidenced by the complete regression of the mass following 24 weeks of anti-TB chemotherapy. This observation underscores the critical importance of promptly initiating anti-TB treatment in individuals suspected of having cardiac TB, especially in those who do not exhibit severe valve dysfunction, both as a diagnostic and management tool, in order to avoid potential complications and enhance patient outcomes. Additionally, the utilization of TEE in endocarditis cases where TTE findings are indeterminate can facilitate timely and accurate diagnosis of cardiac TB.