1. Background
Migraine is a chronic condition characterized by moderate to severe headache attacks that adversely affect individual and social quality of life (1). It is a common disorder that imposes significant costs on community health systems due to declines in academic and occupational performance among those affected (2). Managing migraine presents two main challenges: Determining who should receive preventive treatment and choosing the appropriate prophylactic medication. Given the chronic nature of this disease, it is crucial to find medications that are more effective and have fewer side effects. Understanding the pathophysiology of migraine is extremely beneficial in this context (3).
Two primary mechanisms, vascular and neuronal, are involved in the pathophysiology of migraine (4). Migraine is considered a disorder of homeostasis, with attacks triggered by improper vascular or neuronal responses of the nervous system to internal and external stimuli (5, 6). Thus, migraine is a complex disease (7). Conditions such as comorbid sleep disorders, depression, and anxiety often trigger headaches, and treating these conditions can aid in migraine management (8, 9). Additionally, several biogenic amines, neuropeptides, and their receptors play roles in the neuronal and vascular mechanisms underlying migraine attacks (10).
Calcitonin gene-related peptide (CGRP) is present in both the central nervous system (CNS) and the peripheral sensory nerves originating from the trigeminal ganglion (11). There is growing evidence that CGRP plays a crucial role in increasing environmental sensitivity and associated pain (12) and possesses proinflammatory properties that contribute to pain during migraine attacks (13).
Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine synthesized in the CNS and acts as a neurotransmitter. Its receptors are critical in the pathophysiology of migraine, regulating cerebral artery tone and modulating pain transmission through the trigeminal system (14). The 5-HT2c receptor, which is widely present in the CNS, has a vasoconstrictor effect and facilitates pain transfer in sensory nerves, making it a target for controlling migraine headaches (15).
Agomelatine, a synthetic analog of the melatonin hormone, exhibits similar pharmacodynamics, including stimulation of melatonin receptors (MT1 and MT2) and inhibition of the 5HT2c receptor. Initially used to treat sleep cycle disorders, depression, and anxiety (16-18), agomelatine also plays a role in modulating CGRP secretion via MT1 and MT2 receptors and inhibiting the 5HT2c receptor, making it noteworthy in migraine management (19). Due to its low side effects, minimal interactions with other medications, and good tolerability (20), agomelatine is considered an alternative to conventional migraine preventive treatments.
Here, we detail the study protocol for the agomelatine trial in episodic migraine (EM) without aura. This is a three-month, parallel, randomized, triple-blind, placebo-controlled trial that evaluates the effectiveness of agomelatine in reducing the severity and frequency of attacks in adults aged 18 to 60 years with EM without aura.
2. Objectives
Although agomelatine has been used to treat major depressive disorder (21), generalized anxiety disorder (18, 22), seasonal affective disorder (23), alcohol dependence (24), and to reduce neuropathic pain (25), our study is among the first to explore its use as a maintenance agent for migraine control. A rationale for using this medication in the study includes its chronobiotic actions mediated by MT1 and MT2 receptors (24) and its role as a 5-HT2C receptor antagonist, which facilitates indirect norepinephrine release (25). In this study, we focus on patients aged 18-60 because agomelatine's bioavailability is relatively low (less than 5% at the effective oral dose) due to its high first-pass metabolism (24). We also include patients who have not yet received maintenance treatment, targeting EM specifically, as patients with chronic migraine (CM) are more likely to have already undergone maintenance therapy. The primary objective of this study is to assess the impact of 25 mg/day oral agomelatine on reducing the frequency and severity of migraines compared with a placebo over a three-month intervention period among adults with EM.
3. Methods
3.1. Design
This study follows the SPIRIT protocol, which provides guidelines for randomized trials (26, 27).
3.2. Trial Design
This study employs a triple-blinded, parallel, randomized controlled design and is conducted in two general referral hospitals associated with Tehran University of Medical Sciences. It aims to evaluate the effectiveness of oral agomelatine in patients aged 18 - 60 years with EMs without aura. Episodic migraine is defined as having 0 to 14 headache days per month, whereas CM involves 15 or more headache days per month. The focus is on patients who seek headache treatment and have not received any maintenance treatment previously. The choice to focus on migraines without aura is due to the unique heritability of migraine with aura, its higher average platelet volume, and its stronger association with various conditions, including stroke, which could lead to different responses to acute and preventative treatments (3, 4). Participants must not have any other chronic diseases such as cardiac or liver disease, nor should they have sleep disorders, anxiety disorders, depression, or endocrine diseases. Additionally, female participants should not be pregnant or breastfeeding. The trial is registered with the Iran Clinical Trials Registry (IRCT20230303057599N1).
3.3. Study Setting and Recruitment
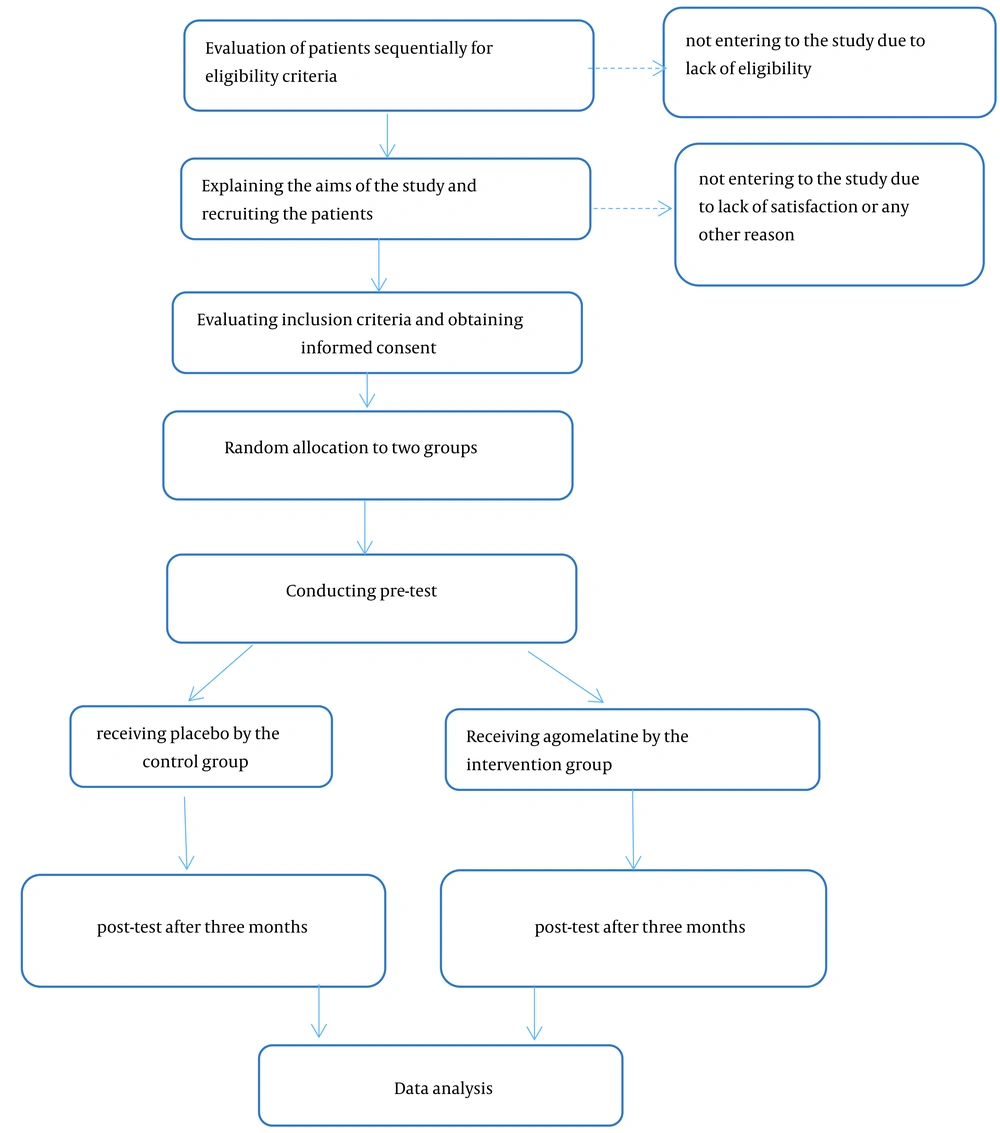
The study takes place at the clinics of general referral hospitals affiliated with Tehran University of Medical Sciences in Tehran, Iran. All patients participating in the study are evaluated for eligibility. After the study's purpose, phases, and duration are explained, informed consent is obtained from each patient who agrees to participate. To achieve the target sample size, potential participants are recruited sequentially as they visit for headache consultations at the clinics. They are informed that participation is voluntary and that they may withdraw from the study at any time without needing to justify their decision.
All study phases will be conducted ethically, with comprehensive information provided to the patients. If they agree to participate, they will sign an informed consent form. Additionally, each patient is assured that if they withdraw from the study, their treatment process will not be interrupted, and standard treatment will continue.
Upon entry, each patient is randomly assigned to either the intervention or control group using a random number table. After randomization, and before the intervention and again three months later, all participants will be evaluated for the frequency and severity of headaches, the number of headache days per month, and using the migraine disability assessment (MIDAS) questionnaire. The control group is of the waiting type, meaning that if agomelatine proves effective, this group will also receive the drug after the trial's completion.
3.4. Eligibility Criteria
Inclusion criteria included:
- Being aged 18 - 60
- Having EM without aura
- Not having received previous maintenance treatment
- Not having chronic diseases such as heart or liver conditions that necessitate ongoing medication use
Exclusion criteria included:
- Reluctance to continue taking medication
- Failure to meet the inclusion criteria
- Concurrent participation in other studies
3.5. Sample Size
To determine the sample size for this study, we reviewed relevant interventional studies published in reputable journals in the field. We first considered headache severity as a variable. Based on a study examining the impact of agomelatine on migraines (28) and the variation in headache severity before (7.8 ± 1.1 points) and after treatment (6.7 ± 1.8 points), with an alpha level of 0.05, a test power of 80% (type 2 error rate of 20%), and accounting for a 10% dropout rate, the sample size was calculated using statistical software for interventional studies. It was determined that a total of 50 patients would be required to participate.
Initially, patients with migraines are assessed to determine if they meet the inclusion criteria. Those deemed suitable and who agree to participate by providing informed consent will be assigned to either the control group or the intervention group. Based on our experience, we estimate that approximately 350 - 450 patients will need to be evaluated to include the necessary number of eligible participants. The sample size for each group is projected to be around 50 individuals, taking into consideration the findings from previous studies, with an alpha of 5% and a beta of 20%. Both the intervention and control groups will consist of an equal number of participants to maximize the power of the statistical test.
3.6. Study Intervention
3.6.1. Intervention Description and Adherence
In this parallel single-phase study, eligible patients are asked to provide written consent and are enrolled in the randomized controlled trial (RCT) if they meet the inclusion criteria. After obtaining informed consent, each patient is randomly assigned to either the intervention or control group using a random number table. In the current study, only the principal researcher (M. Sh.S) knows the type of drugs. The prescriber of the medicine, as well as the individuals responsible for data collection before and after the intervention and the statistical analyst, are unaware of the group assignments.
During the first visit, all patients are asked to provide basic demographic information and fill out a migraine history questionnaire, which includes information on migraine severity, frequency per month, duration, and MIDAS score. Patients are also taught how to fill out migraine records during this visit.
Patients in the intervention group will be prescribed a 25 mg tablet of agomelatine, while patients in the control group will receive a placebo in the form of a vitamin B1 tablet that looks identical to agomelatine. All patients are advised to take pain-relieving medications such as triptans or NSAIDs at the onset of headaches and to follow recommendations for migraine prevention, including dietary modifications and sleep correction. Both groups receive equal education on these matters from the doctor. Each patient visits monthly and receives 30 tablets in addition to their routine medication for use at the onset of a headache. These visits are conducted by a doctor who is unaware of the assigned group of patients. All patients will be taught to use the tablets at night.
The education provided to both groups includes instructions on how to prevent migraines and how to properly fill out the provided form. This form contains necessary information about the timing, date, severity, and duration of headaches. The intervention is performed for each patient over three months, after which a posttest is conducted. Based on the results of the posttest, agomelatine may be prescribed for the control group. Patients in both groups are contacted by phone every month if they do not come for follow-up visits. Figure 1 illustrates the stages of the RCT.
3.6.2. Supply of Intervention
The tablets, both agomelatine and vitamin B1, are sourced from a reputable pharmaceutical company that has been importing and exporting drugs for 25 years and manufacturing drugs in Iran for 15 years. They are light yellow and are packaged in sheets of 10. The tablets are labeled A and B, but only the principal investigator knows which type each label represents.
3.6.3. Withdrawal of Interventions
Patients who wish to withdraw from the study are advised to inform the doctor who prescribed their tablets. Before joining the study, patients are informed that participation is entirely voluntary and that they can withdraw at any time without providing a reason. Furthermore, they are reassured that their treatment will continue uninterrupted with routine care provided. However, patient data will continue to be analyzed for the duration of their participation in the study.
3.7. Related Concomitant Care During the Clinical Trial
Patients are advised to inform their prescriber about any medication they take, even if it is part of their regular regimen. Additionally, participants should not be involved in another study simultaneously.
3.8. Outcomes
The purpose of this clinical trial is to determine whether agomelatine oral tablets can reduce the frequency and severity of migraine attacks in adults with EM. Data are collected through questionnaires that measure headache frequency and severity, the number of headache days per month, and the MIDAS score. Research colleagues conduct interviews with patients and review their diaries to collect this information.
3.9. Statistical Analysis
Data analysis is conducted using SPSS Statistics for Windows, Version 16. Any P-values below 0.05 are deemed statistically significant. The results are obtained using descriptive and inferential statistics, such as mean, standard deviation, and comparing the main variables of the study with independent and paired t-tests. Continuous data will be reported as a group mean ± standard deviation (SD), and categorical data will be reported as counts (percentages). The data analyst will not be informed of group allocation until the study's conclusion.
4. Discussion and Conclusions
The rationale for this study is grounded in the specific effects of agomelatine on the trigeminal vascular system, as well as its positive results in treating sleep disorders, depression, and anxiety (16-19). This study was designed due to a lack of sufficient clinical trials on the use of agomelatine as a preventive therapy for migraines.
Advances in understanding the role of melatonin receptors in ameliorating chronobiological disorders, such as circadian rhythm sleep disturbances, have led to the development of a new class of melatonin agonists (29). Agomelatine is structurally similar to melatonin and, in addition to being a potent agonist of MT1 and MT2 melatonin receptors, it is a selective 5-HT2c receptor antagonist. Due to its synergistic action on melatonergic and 5-HT2c receptors, agomelatine possesses anti-inflammatory (30), vasodilatory (31), pain modulatory (32), and chronobiotic properties (24). Thus, it could be potentially effective in the preventive treatment of migraines. With this multimodal mechanism, agomelatine may eliminate the need for multi-drug therapies, which typically increase the risk of side effects.
Based on available evidence, the effective dose for managing migraines is considered to be 25 mg per day (19), and at this dosage, side effects are expected to be mild and tolerable (33). The most significant complications reported for agomelatine are hepatotoxicity, which is dose-dependent and age-dependent (34). In this study, hepatotoxicity is expected to be minimized due to the short duration of the intervention (3 months), the exclusion of patients over 60 years, and those with a history of liver disease. No serious drug interactions with agomelatine have been reported (35). Therefore, the administration of agomelatine appears to be safe with controlled monitoring of liver enzymes.
Agomelatine, with its favorable side-effect profile compared to conventional migraine prophylactic medications, can be considered an alternative preventive treatment. In this trial, we include patients suffering from migraines without aura and use subjective measures, including diaries and questionnaires, to evaluate the treatment outcome.
According to the evidence, the risk of ischemic stroke increases significantly in migraine with aura (36). Therefore, preventive treatments should be selected not only to decrease the severity and frequency of migraine attacks but also to reduce the risk of cerebrovascular complications. In future studies, the effects of agomelatine on the cerebrovascular system should be evaluated more precisely through objective measures such as imaging of the microvasculature, especially for use as a preventive treatment for migraine with aura in place of conventional medications.
One of the strengths of this study is its randomized design, which ensures an equal number of participants in each group and gives each individual an equal chance of being placed in any group. Another strength is the concealment of group assignments so that neither the prescriber nor the data collector knows which group a participant will be in next. The choice of agomelatine and its dosage are based on previous studies and a thorough review of the literature. Additionally, the control group in this study is a waiting group, meaning that they will receive agomelatine following the posttest if it proves effective. The triple blinding and monthly follow-ups through phone calls to collect data and remind patients to take their medication are further advantages that help prevent participant loss.
In this trial, the effectiveness of agomelatine in preventing migraines is compared to that of a placebo in the age group of 18 to 60 years. For a more comprehensive understanding of the side effect profile, further studies should test agomelatine in adolescents and patients older than 60. Additionally, to better understand the advantages and disadvantages of different preventive treatments, clinical trials comparing the effectiveness of agomelatine with other maintenance medications for migraine control are recommended.