1. Background
Acute lymphoblastic leukemia (ALL) is a clinically heterogeneous disorder characterized by the uncontrolled proliferation of malignant lymphoid cells, which leads to a block in differentiation and subsequent accumulation in the bone marrow, peripheral blood, and other tissues (1). According to the Iraqi cancer registry, leukemia ranks third among cancers in adults, affecting both males and females, with a prevalence of 4.8 per 100,000 population. In the pediatric age group, leukemia is the leading cancer type (2).
Although ALL is the most common type of leukemia in children, it exhibits a bimodal age distribution, with two peaks: One in childhood and another around age 50 (3). Various factors affect the treatment modalities and clinical outcomes in ALL, with age being a significant prognostic factor. Clinical outcomes differ between adults and pediatric patients, even when the treatment approaches are similar (4).
Immunophenotyping is a crucial tool for diagnosing ALL. Its role has expanded to include prognosis and serving as a surrogate for genetic alterations, which are currently the most important determinants of prognosis (5). Treatment decisions are heavily influenced by the immunophenotypic profile. Certain subtypes of ALL show enhanced responsiveness to targeted therapies or specific chemotherapy regimens, such as Rituximab for CD20-positive patients and Ofatumumab for those targeting CD52, as reviewed by (6). Additionally, immunophenotyping helps in risk stratification by identifying patients at higher risk for relapse or treatment failure (7).
Comparative assessments of ALL between adults and children can provide valuable insights into potential variations in disease mechanisms and therapeutic responses. This can facilitate advancements in research and the development of new therapeutic strategies tailored to each demographic. These differences also have significant clinical implications, affecting treatment efficacy, relapse rates, and overall survival. Understanding the immunophenotypic variations between pediatric and adult ALL could help tailor chemotherapy regimens to specific age groups, potentially improving patient outcomes (4).
2. Objectives
This study aims to identify the immunophenotyping profiles of ALL in adult and pediatric populations and to explore whether antigen profile differences exist between the two groups.
3. Methods
This cross-sectional study utilized archival data from 131 newly diagnosed ALL patients admitted to the Hematology Unit at Baghdad Teaching Hospital or the Children's Welfare Hospital at the Medical City campus (for the pediatric group) between May 2014 and May 2015. Patients with relapsed ALL or secondary ALL (transformed from non-Hodgkin lymphoma) were excluded from the study.
For all patients, the diagnosis was confirmed through morphology and immunophenotyping. Ethical approval for the study was obtained from Baghdad Teaching Hospital (Approval No. EAC-253664). Peripheral blood and/or bone marrow samples were collected in EDTA tubes. A complete blood count was performed using a Beckman Coulter hematology analyzer. Morphological examination of Leishman-stained blood smears was carried out by two hematopathologists. Immunophenotypic evaluation was conducted using six-color flow cytometry (BD-FACS-Canto II System, Becton Dickinson, Belgium) at the Nursing Home Hospital within the Medical City campus. Samples were analyzed within 24 hours of collection using a panel of fluorochrome-labeled antibodies, including the following fluorochromes: Fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (Per-CP), and allophycocyanin (APC). Antibodies included CD45, CD1a, CD2, CD3, CD4, CD5, CD7, CD8, CD19, CD11b, CD13, CD14, CD15, CD16, CD20, CD33, CD34, CD56, CD64, CD117, and HLA-DR.
For cytoplasmic antigens, samples were treated with a permeabilizing solution (BD Cytofix/Cytoperm, Fisher Scientific, UK), followed by labeling with 6 µL of fluorochrome-conjugated antibodies for MPO, TdT, cCD3, and cCD79a, following the standard operating procedure. The samples were incubated for 15 minutes in the dark, followed by the addition of 2 mL of lysing solution and further incubation for 10 minutes. The tubes were centrifuged at 1500 rpm for 5 minutes, the supernatant was discarded, and the cells were washed twice with 2 mL of BD Cell Wash. The cell pellet was resuspended in 0.5 mL of CellFIX solution (BD CellFIX, Fisher Scientific, UK) and the sample was ready for acquisition and analysis.
Data acquisition was performed using BD FacsDiva software (BD, Belgium). Gating was done using CD45 expression versus side scatter analysis. Surface antigens were considered positive if their expression exceeded 20%, while cytoplasmic antigens, CD34, and CD117 had a threshold limit of 10%.
Statistical analysis was conducted using SPSS version 20 software (IBM Corp., Armonk, NY, USA). The chi-Square test was used to analyze nominal data, with a P-value of less than 0.05 considered statistically significant.
4. Results
A total of 131 newly diagnosed ALL patients were included in the study, with 64 adult patients and 67 pediatric patients (maximum age of 16 years). The mean age for the adult group was 32.38 years, while the mean age for the pediatric group was 6.80 years. In the adult group, males comprised 40 (62.5%) of the patients, yielding a male-to-female ratio of 1.6:1. In the pediatric group, males constituted 44 (65.7%) of the patients, with a male-to-female ratio of 1.9:1.
The B-ALL phenotype was prevalent in 44 (68.8%) of the adult patients compared to 51 (76.1%) in the pediatric group. Despite the higher number of B-ALL patients in the pediatric group, the difference was not statistically significant. CD10 expression was observed in 49 (76.6%) of adult ALL cases, primarily in B-ALL. Lower expression of CD10 was noted in the pediatric group, particularly in T-ALL, and this difference was statistically significant (P = 0.039) (Table 1).
| Variables | Adult Group; (n = 64) | Pediatric Group; (n = 67) | P-Value |
|---|---|---|---|
| Age | 32.38 ± 14 | 6.8 ± 4.7 | 0.72 |
| Male: Female | 1.6: 1 | 1.9: 1 | |
| ALL subtypes | - | - | 0.43 |
| B ALL | 44 (68.7) | 51 (76.1) | |
| T ALL | 20 (31.2) | 16 (23.8) | |
| Aberrant myeloid Ag expression | 29 (45.3) | 30 (44.8) | 0.367 |
| B ALL | 19 (29.7) | 26 (38.8) | |
| T ALL | 10 (15.6) | 4 (6) | |
| CD10 Total | 49 (76.6) | 47(70.1) | 0.486 |
| B-ALL | 39 (60.9) | 44 (65.7) | 0.644 |
| T-ALL | 10 (15.6) | 3 (4.5) | 0.039 |
| CD34 expression | 44 (68.7) | 49 (73.1) | 0.582 |
| CD38 expression | 46 (71.8) | 54 (80.6) | 0.24 |
| Cytoplasmic TdT | 57 (89.1) | 52 (77.6) | 0.08 |
| CD56 | 4 (6.3) | 2 (2.9) | 0.372 |
Immunophenotyping Features of Acute Lymphoblastic Leukemia in the Studied Groups. a
Aberrant myeloid antigen expression was seen in 29 (45.3%) of the adult cases, of which 19 (43.2%) were B-ALL, with the remainder being T-ALL. Similarly, in the pediatric group, aberrant myeloid expression was found in 30 (44.8%) of cases, with 26 (50.9%) being B-ALL, and the remainder being T-ALL (Table 1).
The most commonly expressed aberrant myeloid antigen in adult ALL cases were CD33, followed by CD13. In the pediatric group, CD13 was the most commonly expressed, followed by CD33. The frequency of aberrant myeloid antigen expression in both groups is summarized in Table 2. No significant differences were found between the two groups for any of the aberrant antigens (Table 2).
| Myeloid Markers | Adult ALL (n = 64) | Pediatric ALL (n = 67) | P-Value |
|---|---|---|---|
| CD13 | 15 (23) | 18 (27) | 0.651 |
| CD33 | 18 (28) | 12 (18) | 0.164 |
| CD16 | 4 (6) | 11 (16) | 0.068 |
| CD64 | 0 (0) | 1 (1) | NA |
| CD117 | 3 (5) | 2 (3) | 0.611 |
| CD15 | 1 (2) | 0 (0) | NA |
| CD11b | 4 (6) | 3 (4) | 0.652 |
The Frequency of Aberrant Myeloid Ag Expression in Study Groups a
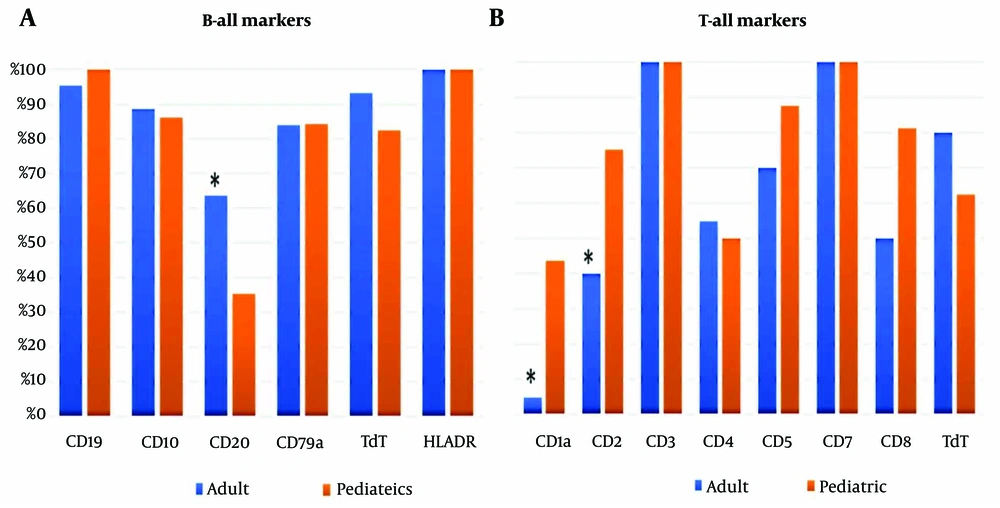
HLA-DR was the most frequently expressed marker in both adult and pediatric patients, with 100% expression, followed by CD19 and cTdT. A significant difference in CD20 expression between adults and pediatric patients was observed (P = 0.006), as shown in Figure 1A.
In T-ALL cases, CD3 and CD7 were expressed in 100% of cases in both groups. However, there were significant differences in the expression of CD1a and CD2 between the adult and pediatric groups, with P-values of 0.007 and 0.05, respectively (Figure 1B).
T-cell antigens were aberrantly expressed in 5 pediatric B-ALL cases, including CD4, CD1a, CD5, and CD2. In contrast, only 2 adult B-ALL cases showed aberrant expression of CD2 and CD4. Aberrant B-cell antigen expression in T-ALL was observed in three adult T-ALL cases and one pediatric T-ALL case, with aberrant expression of CD79a. Neither CD19 nor CD20 were expressed in any of the cases.
5. Discussion
ALL is a heterogeneous disease. Prognosis and personalized therapy are determined by risk stratification, immunophenotype, cytogenetics, and molecular markers. However, in Iraq, cytogenetic studies are not routinely conducted due to limited resources, making diagnosis and subtyping largely dependent on immunophenotyping (8, 9). This study examined the differences in immunophenotypes between adults and pediatric ALL patients at the time of diagnosis.
T-cell lineage ALL is classified as a high-risk category. In our study, the prevalence was higher than reported in previous studies for both adult (31.2%) and pediatric (23.8%) groups. Other local and international studies have reported lower incidence rates, ranging between 20–25% in adults (10, 11) and about 15% in pediatric ALL cases (12).
When comparing the immunophenotypes of adult and pediatric B-ALL patients, we found that CD20 expression was significantly higher in adults (64% vs. 35%), consistent with other studies (13, 14). In adults, CD20 expression is associated with inferior survival, but this is not observed in children (15). A study involving 353 children with B-cell precursor ALL found that 48% of patients expressed CD20, and those who did tended to have better treatment outcomes compared to those without CD20 expression (15, 16). Anti-CD20 therapies such as rituximab, obinutuzumab, and atumumab have shown promise, even with low CD20 expression, by reducing recurrence and improving survival (17). Several trials have suggested that adding rituximab to aggressive treatments for CD20+ ALL can improve outcomes and may reduce the need for bone marrow transplants in pediatric patients (18).
In T-ALL, a notable difference between adult and pediatric cases was observed in the expression of CD1a and CD2. CD1a is a surface glycoprotein found in cortical T-ALL and Langerhans cells, but absent in growing and mature T cells, making it a potential therapeutic target for T-ALL (19). In this study, over 50% of pediatric T-ALL cases expressed CD1a, compared to less than 5% in adults. CD1a+ cortical T-ALL is a common immunophenotype in newly diagnosed T-ALL, although its clinical significance remains debated. Some studies suggest that CD1a+ patients have a more favorable prognosis, with higher complete remission and lower recurrence rates (20). However, other studies have not demonstrated a significant impact (12).
CD1a has emerged as a new CAR-T cell target, showing strong cytotoxic effects in preclinical research, including xenograft models. This approach was effective in a small percentage of relapsed CD1a+ patients, but resistance mechanisms such as CD1a deletion or the selection of a CD1a- subclone have been suggested as potential challenges (6).
CD2, a pan-T-cell antigen, is typically expressed early in the maturation of T cells in the thymus and is present on all subsets of mature T cells (21). Together with its ligand, CD58, CD2 co-stimulation activates T cells and signals the T cell receptors (22). In our study, three-quarters of pediatric T-ALL cases expressed CD2, compared to only 40% of adults. Li et al. reported a mean CD2 expression rate in B-ALL of 0.84% ± 0.67%. They also noted that circulating CD2- T cells were increased in the peripheral blood of pediatric AML and B-ALL patients, which was associated with KMT2A gene rearrangement and an unfavorable outcome (21). A recent study introduced an allogenic CD2-targeting CAR-T cell (UCART2), a novel therapy that prevents fratricide and life-threatening GvHD by biallelic deletion of CD2 and TRAC. UCART2 effectively kills CD2+ primary human T-ALL and CD2+ T-ALL and CTCL cell lines in vitro and in vivo. Pre-clinical data showed that UCART2, when combined with rhIL-7-hyFc, results in curative and durable therapeutic responses (23).
CD10, a common acute lymphoblastic leukemia antigen (CALLA), is an early marker of B-cell differentiation and is expressed in most ALL cases. Research has shown that the majority of these cases involve childhood precursor B cell malignancies with a favorable prognosis (24). In our study, CD10 was more frequently expressed in adults (76.6%) compared to pediatric patients (70.1%), which is lower than previously reported by other studies (25). Negative CD10 expression in B-ALL has been correlated with MLL rearrangement, which is associated with a poor prognosis (14). A switchable UniCAR system has been developed to explore CD10 as a therapeutic target for B-ALL. The UniCAR platform links T cells to tumor cells via target modules (TMs). Studies found that UniCAR T cells with anti-CD10 TM effectively killed B-ALL cell lines and patient-derived blasts, suggesting CD10 as a potential therapeutic target (26).
Aberrant phenotypes, characterized by the coexpression of non-lineage markers, occur in ALL with varying frequency and have controversial clinical significance (27). In our study, we observed a relatively higher rate of aberrant myeloid antigen expression in adult B-ALL (43.2%) and T-ALL (50%) compared to previous studies, which reported rates of 38% and 24%, respectively (27, 28). While several studies suggest that these aberrant phenotypes have an uncertain impact on patient outcomes (30), the recently defined Early T-cell Precursor ALL (ETP-ALL), which expresses myeloid and/or stem cell markers, has been associated with a poor prognosis (29). The variability in aberrant phenotype expression could be attributed to technical inconsistencies, including variations in monoclonal antibody binding, as well as the lack of standardized diagnostic criteria, which may lead to the misclassification of mixed phenotypes (14).
This study has some limitations. The absence of electronic archiving made it difficult to obtain follow-up data for comparing patient outcomes and assessing the significance of phenotypic differences on disease relapse.
5.1. Conclusions
Our results demonstrated an increase in T-cell lineage involvement and aberrant antigen expression in both adult and pediatric ALL. Adults with ALL tend to exhibit higher levels of CD20 and lower levels of CD1a and CD2 compared to children, markers that are associated with poorer prognoses.