1. Background
Breast cancer is the most common cancer globally and a leading cause of death among women in both economically developed and developing countries, accounting for 12.5% of all new annual cancer cases worldwide (1). According to the latest report by the World Health Organization (WHO), it is estimated that in 2020, there were 2.3 million women diagnosed with breast cancer and 685,000 deaths globally. By the end of 2020, there were approximately 7.8 million women alive who had been diagnosed with breast cancer within the past five years, making it the most prevalent cancer worldwide (2). Breast cancer results in more lost disability-adjusted life years (DALYs) globally than any other type of cancer (3). While breast cancer is rare in men (0.5 - 1% of all cases), it occurs much more frequently in women, making female gender the strongest risk factor for the disease. Breast cancer can develop in women of any age after puberty but rates increase later in life (2).
In Iran, breast cancer is the most prevalent malignancy among women, with an incidence rate of 22 per 100,000 (4). Studies indicate that the age of onset in Iranian women is relatively lower compared to global averages. More than 70% of the patients have a tumor size larger than 2 cm and about 63% exhibit lymph node involvement at the time of diagnosis (5).
Breast cancer is a heterogeneous and complex disease, encompassing multiple tumor entities each associated with different histological types and distinct biological characteristics and clinical behaviors. Due to the genetic and clinical heterogeneity of the disease, breast cancer has various subtypes. Over the years, the classification of breast cancer subtypes has evolved significantly. The most common and widely accepted method for classifying breast carcinoma is based on immunohistochemical characteristics. According to this method, breast cancers are commonly categorized into four main groups: Estrogen receptor positive (ER+), progesterone receptor positive (PR+), human epidermal growth factor receptor 2 positive (HER2+), and triple-negative breast cancer (TNBC), which lacks expression of ER, PR, and HER2 receptors (6).
Molecular categorization of breast cancer is pivotal for identifying patients who may benefit from targeted therapies, such as hormone therapy for ER+ and PR+ cancers, and anti-HER2 therapy for HER2+ cancers (7). Additionally, breast tumors are classified based on the identification of differentially expressed genes, long non-coding RNAs, and RNA binding proteins specific to each subtype. For example, specific genes like RASDF7 are linked to luminal A, DCTPP1 to luminal B, and genes like KLC3, NAG3, DHRS11, and TMEM98 to HER2, while ABDHD14A and ADSSL1 are associated with TNBC. These genes provide preliminary evidence for identifying new prognostic biomarkers and therapeutic targets for each subtype.
Complementary DNA microarray research on breast cancer tissue further categorizes these cancers into four primary molecular subtypes: Luminal A, luminal B, triple negative/basal-like, and HER-2. luminal A breast cancer, which is estrogen and progesterone receptor positive, HER2 negative, and has low levels of the protein Ki-67, tends to grow more slowly, is of lower grade, and generally has a good prognosis. In contrast, luminal B breast cancer is estrogen receptor positive and HER2 negative but either has high levels of Ki-67, indicating faster cancer cell growth, or is progesterone receptor negative (8-10).
According to existing evidence, factors such as histological type, tumor size, grade, lymph node involvement, and the status of ER α, PR, and HER-2 receptor are all related to the prognosis and the likelihood of therapeutic response (11). Previous studies have identified Ki-67 as another prognostic biomarker in invasive breast cancer, with its expression strongly associated with cancer proliferation. The primary objective of cancer classification is to accurately diagnose the disease and predict tumor behavior to facilitate decision-making in cancer treatment. While traditional categorization of breast cancer has primarily relied on clinical and pathological characteristics, as well as routine biomarker evaluation, it may not adequately reflect the diverse clinical trajectories of breast cancer. Recent advances in high-throughput technologies have provided crucial insights into the genetic alterations and biological events underlying breast cancer, offering clinicians new strategies for treatment and patient stratification (12).
Although numerous studies have examined the epidemiology of breast cancer in Iran, there is limited information on the histopathological and molecular characteristics of this disease among Iranian patients (13, 14). In the Kurdistan province and among the Kurdish population, a comprehensive study on the molecular and histopathological characteristics of breast cancer has yet to be conducted up to the time of this research. A study from northeastern Iran highlighted an ethnic disparity in breast cancer patterns (15).
2. Objectives
Considering the significant role that histopathological and molecular characteristics play in the management and mitigation of breast cancer risks and complications, and the current lack of detailed information in our setting, this study aimed to investigate the frequency of histopathological and molecular types of malignant breast tumors among the Kurdish population during the years 2019 - 2021.
3. Methods
3.1. Study Design, Setting and Patients
In this descriptive-analytical cross-sectional study, we examined all pathology reports from patients who underwent breast biopsies in Sanandaj city, a central location in Kurdistan province, northwest Iran, from 2019 to 2021. This included approximately 1,631 reports. The majority of the samples (83.9%) were obtained using the core-needle biopsy (CNB) method, 15.9% by surgical excision, and only one case by fine-needle aspiration (FNA). Out of these reports, 620 (38%) were related to malignant breast masses. The specimens had already undergone fixation, processing, sectioning, and staining to prepare pathology slides. The final diagnosis and histopathological characteristics were determined by an experienced pathologist. Additionally, antigen retrieval, blocking, antibody labeling, and visualization steps were carried out by a skilled laboratory technician, followed by reporting of the immunohistochemical results. Due to the low number of Paget's disease cases (12 or 2%), these were excluded from the study. Also excluded were 10 cases (1.5%) where molecular or histopathological characteristics were not available. Ultimately, 597 reports (36%) of the remaining malignant cases were included in the analysis.
3.2. Variables and Data Collection Method
For this study, the age and sex of each patient, the type of pathology and its characteristics, and the molecular results of the sample [based on the immunohistochemistry (IHC) method] were recorded and extracted from the laboratory software, which utilized special codings. The data were organized according to the type of pathology (Ductal, Lobular, Mucinous, Papillary, Metaplastic) and molecular markers (ER, PR, HER2, Ki-67) along with their characteristics.
In current practice, the expressions of ER, PR, and HER2 were assessed using immunohistochemical staining to evaluate protein expression. The histological grade of the cases was determined using the Elston-Ellis modification of the Scarff-Bloom-Richardson grading system, which includes the summation of scores for tubular formation, nuclear pleomorphism, and mitotic counts (16). Additionally, the expressions of ER, PR, HER2, and the Ki-67 index were evaluated through immunohistochemical analysis.
To ensure data accuracy, after initially sorting the data by the patients' first and last names and residential addresses, any duplicate records were identified and excluded. Also excluded were patients who resided in another city, those who had previously been diagnosed with breast cancer, or those who had referred for chemotherapy, radiotherapy, or follow-up post-surgery. This meticulous approach was employed to maintain the integrity and specificity of the study's dataset to the target population of Sanandaj city.
3.3. Ethical Considerations
Given that this study employed a retrospective approach, the ethical implications and issues were minimal. To protect patient identity and ensure confidentiality, each patient was assigned a specific code during data collection. The study proposal was evaluated and approved by the ethics committee at Kurdistan University of Medical Sciences, receiving the ethics code: IR.MUK.REC.1400.140.
In instances where necessary data were missing, we contacted each patient to explain the study's objectives and procedures before data collection commenced. For these cases, written informed consent was obtained from each participant prior to gathering their data, ensuring that all participants were fully informed and voluntarily contributing to the research. This process not only adhered to ethical standards but also enhanced the integrity and reliability of the study findings.
3.4. Statistical Analysis
All statistical analyses were performed using SPSS 26.0 software (SPSS Inc., Chicago, Illinois, USA). Descriptive quantitative variables were expressed as means ± standard deviation (SD), while qualitative variables were presented in terms of frequency (%). To assess the relationships between the study variables, chi-square and Fisher's exact tests were applied. These methods facilitated the exploration of potential correlations and differences within the data, providing a robust statistical foundation for interpreting the study findings.
4. Results
Of the 597 malignant cases analyzed, 591 (99.0%) were women and 6 (1.0%) were men, reflecting the significantly higher prevalence of breast cancer among women. The mean age of the participants was 49.73 years, with a standard deviation of 11.88 years. The age range of participants varied considerably, with the oldest patient being 89 years old, diagnosed with infiltrating lobular carcinoma, and the youngest being 25 years old, diagnosed with infiltrating ductal carcinoma. Infiltrating ductal carcinoma was the predominant type, occurring in 82.7% of the patients, indicating its commonality in breast cancer cases. Detailed statistics including the frequency of each histological type of breast cancer, as well as the mean, standard deviation, maximum, and minimum ages of the subjects are summarized in Table 1. This table provides a comprehensive overview of the demographic and pathological characteristics of the study cohort.
| Histopathologic Type | No. (%) | Age (Mean ± SD) | Min | Max |
|---|---|---|---|---|
| Infiltrating ductal carcinoma | 494 (82.7) | 49.40 ± 11.80 | 25 | 86 |
| Infiltrating lobular carcinoma | 49 (8.2) | 49.82 ± 11.95 | 27 | 89 |
| Metaplastic breast cancer | 11 (1.8) | 55.09 ± 10.59 | 41 | 70 |
| Mucinous carcinoma | 8 (1.3) | 44.75 ± 5.04 | 39 | 51 |
| Papillary (intraductal) carcinoma | 35 (5.9) | 53.80 ± 13.56 | 28 | 77 |
As indicated in Table 1, the mean age at the time of cancer detection for cases with mucinous carcinoma was approximately 5, 9, and 11 years younger than for those with infiltrating ductal/lobular, papillary, and metaplastic breast cancers, respectively. Notably, while younger cases (under 30 years) were observed among three pathological types—namely, infiltrating ductal, infiltrating lobular, and papillary carcinomas—no cases under 40 years of age were detected among those with metaplastic breast cancer. Furthermore, the mean age of cancer detection in cases with metaplastic breast cancer was higher than in other types.
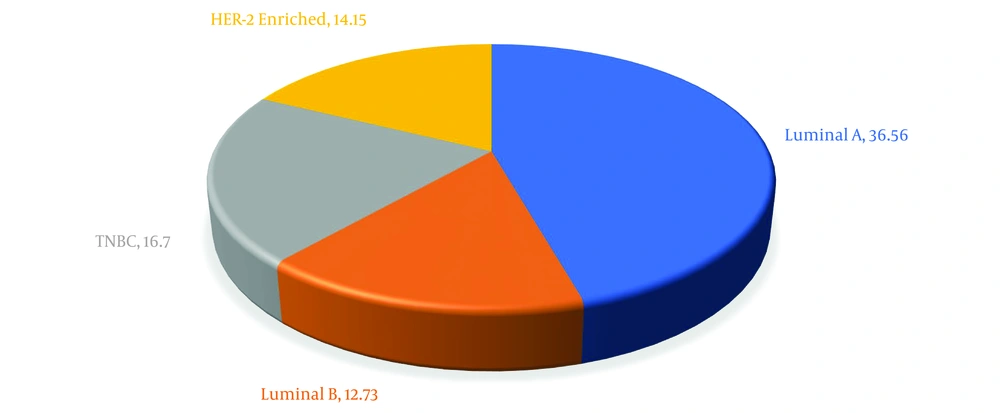
In terms of frequency of malignant breast tumors, luminal A subtype was the most common with 239 cases (36.56%), whereas luminal B subtype had the lowest occurrence with 54 cases (12.73%). Triple-negative breast cancer constituted 16.74% of all cases, highlighting the variation in molecular subtypes among the study cohort. These findings are visually summarized in Figure 1.
Estrogen receptor was positive in 71.4% of cases and negative in 28.6%. Progesterone receptor showed positivity in 63.2% of cases and negativity in 36.8%. HER2 status was positive in 19.4% of cases, equivocal in 14.1%, and negative in 66.5%. Notably, there were no equivocal cases of HER2 in metaplastic breast cancer. Ki-67, a marker of proliferation, was positive in 74.1% of cases and negative in 25.9%.
Table 2 details the association between the pathological types of breast cancer and related biomarkers (ER, PR, HER2, and Ki-67). As highlighted in Table 2, Ki-67 is the only biomarker showing a significant association with pathological types of breast cancer. Specifically, a higher percentage of cases positive for Ki-67 was observed in three types of breast cancer: Infiltrating ductal carcinoma, metaplastic, and papillary (intraductal) carcinoma, compared to other types. This suggests that Ki-67 is an important indicator of tumor aggressiveness in these specific types of breast cancer.
| Variables | ER (n = 475) | P | PR (n = 475) | P | HER-2 (n = 474) | P | Ki-67 (n = 463) | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Equivocal | Positive | Negative | Positive | Negative | |||||
| Infiltrating ductal carcinoma | 276 (69.5) | 121 (30.5) | 0.06 | 246 (62.0) | 151 (38.0) | 0.12 | 59 (14.9) | 80 (20.2) | 257 (64.9) | 0.06 | 301 (78.2) | 84 (21.8) | < 0.001 |
| Infiltrating lobular carcinoma | 28 (80.0) | 7 (20.0) | 25 (71.4) | 10 (28.6) | 1 (2.9) | 3 (8.6) | 31 (88.6) | 11 (31.4) | 24 (68.6) | ||||
| Metaplastic breast cancer | 4 (57.1) | 3 (42.9) | 2 (28.6) | 5 (71.4) | 0 | 3 (42.9) | 4 (57.1) | 5 (71.4) | 2 (28.6) | ||||
| Mucinous carcinoma | 4 (66.7) | 2 (33.3) | 4 (66.7) | 2 (33.3) | 1 (16.7) | 2 (33.3) | 3 (50.0) | 2 (33.3) | 4 (66.7) | ||||
| Papillary (intraductal) carcinoma | 27 (90.0) | 3 (10.0) | 23 (76.7) | 7 (23.3) | 6 (20.0) | 4 (13.3) | 20 (66.7) | 24 (80.0) | 6 (20.0) | ||||
As indicated in Table 3, the grading of breast carcinoma showed a significantly different distribution among all pathological types (P < 0.001). Specifically, 83% of cases with the metaplastic subtype were classified as grade 3 at diagnosis, indicating a high degree of tumor aggressiveness. Conversely, more than 85% of cases with the infiltrating lobular and mucinous subtypes were classified as grade 1, suggesting a lower degree of tumor aggressiveness.
For the papillary subtype, the majority of cases (62.9%) were classified as grade 2, indicating a moderate level of tumor aggressiveness. Additionally, more than 60% of cases with invasive ductal carcinoma and papillary carcinoma were diagnosed at stages 2 and 3, reflecting more advanced disease at the time of diagnosis. This data underscores the variability in aggressiveness and stage at diagnosis across different pathological types of breast cancer.
| Variables | 1 | 2 | 3 | P-Value |
|---|---|---|---|---|
| Infiltrating ductal carcinoma | 168 (36.2) | 200 (43.1) | 96 (20.7) | < 0.001 a |
| Infiltrating lobular carcinoma | 39 (88.6) | 5 (11.4) | 0 | |
| Metaplastic breast cancer | 0 | 1 (16.7) | 5 (83.3) | |
| Mucinous carcinoma | 6 (85.7) | 1 (14.3) | 0 | |
| Papillary (intraductal) carcinoma | 12 (34.3) | 22 (62.9) | 1 (2.9) | |
| Total | 225 (40.5) | 229 (41.2) | 102 (18.3) | - |
a Fisher's exact test.
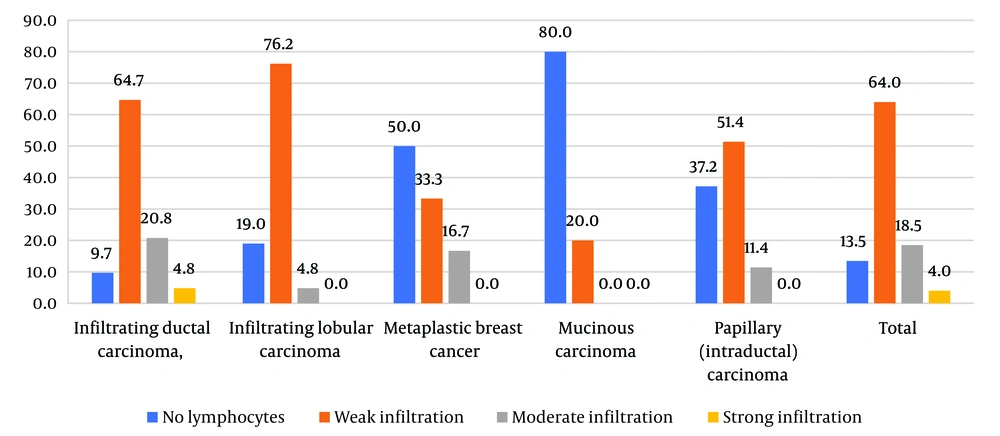
Stromal tumor infiltrating lymphocytes (sTILs), which are associated with a patient's immune response, serve as a validated predictive and prognostic biomarker in non-luminal breast cancer types. According to Figure 2, the presence of sTILs, indicating immune response, is significantly higher in the subtype of infiltrating ductal carcinoma, followed by lobular carcinoma (P < 0.001). The lowest levels of sTILs were observed in the mucinous carcinoma subtype.
Additionally, lymphatic invasion, venous invasion, and perineural invasion were evaluated for each subtype. As summarized in Table 4, the frequency distribution of lymphatic invasion and venous invasion among the studied subtypes did not show significant differences, although venous invasion was not observed in the metaplastic and mucinous carcinoma subtypes. Furthermore, metaplastic carcinoma was the only subtype that exhibited no lymphatic invasion. Perineural invasion was present in 34% of lobular and 10.3% of ductal carcinoma cases, which was significantly different from other types that had no cases with perineural invasion (P < 0.001). This variation underscores the importance of histological subtype in predicting the extent of cancer spread and potential invasion of surrounding tissues.
| Variables | Lymphatic Invasion (n = 563) | P | Venus Invasion (n = 563) | P | Perineural Invasion (n = 560) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | ||||
| Infiltrating ductal carcinoma | 124 (26.5) | 344 (73.5) | 0.16 | 40 (8.5) | 428 (91.5) | 0.52 | 48 (10.3) | 418 (89.7) | < 0.001 |
| Infiltrating lobular carcinoma | 8 (17.0) | 39 (83.0) | 2 (4.3) | 45 (95.7) | 16 (34.0) | 31 (66.0) | |||
| Metaplastic breast cancer | 0 | 6 (100.0) | 0 | 6 (100.0) | 0 | 6 (100.0) | |||
| Mucinous carcinoma | 3 (42.9) | 4 (57.1) | 0 | 7 (100.0) | 0 | 6 (100.0) | |||
| Papillary (intraductal) carcinoma | 12 (34.3) | 23 (65.7) | 5 (14.3) | 30 (85.7) | 0 | 35 (100.0) | |||
a Values are expressed as No. %.
5. Discussion
In this study, we evaluated the distribution of various molecular subtypes of malignant breast tumors among Kurdish patients in Sanandaj, the center of Kurdistan province in Iran, and investigated the differences in clinical and pathological features between these subtypes. Our findings showed that the mean age of the patients was 49 years, closely aligning with the national average of 47.1 years (17). The most common malignant breast tumor identified was infiltrating ductal carcinoma, which accounted for 82.7% of cases, followed by infiltrating lobular carcinoma at 8.2%. Comparatively, a large study in the United States involving 135,517 women diagnosed with breast cancer reported frequencies of 76.0% for infiltrating ductal carcinoma and 8.0% for infiltrating lobular carcinoma (18). Similarly, Maffuz-Aziz et al. observed these two pathological types at 79.8% and 7.8%, respectively (19), which aligns with our findings.
The luminal A subtype was the most prevalent in our study, consistent with results from a retrospective study by Fatma Khinaifis at King Abdul Aziz University Hospital in Saudi Arabia, which found luminal A to be the dominant subtype at 58.5% (20). Our results regarding the distribution of molecular subtypes are also in agreement with findings from other studies conducted in various Asian and Western countries (21-24). These studies consistently found luminal A to be the most frequent subtype, with minor geographical variations possibly attributable to genetic factors, environmental variables, and/or technological disparities.
In contrast to our findings, Al Tamimi et al. reported that more than half of their cases (52.8%) were triple negative, while luminal tumors accounted for only 28.5% (25). This discrepancy highlights the potential influence of regional and ethnic differences on the prevalence and distribution of molecular subtypes of breast cancer.
Our findings show that the expression of ER, PR, HER-2, and Ki-67 markers were positive in 71.4%, 36.1%, 19.4%, and 74.2% of tumors, respectively. These results align closely with those reported by Zhao et al. (26) and Maffuz-Aziz et al. (19), suggesting that the molecular behavior of breast tumors exhibits little variability across different regions. However, Nafissi et al., in their review, noted that the epidemiology and histopathology of breast cancer in Iran show some differences compared to neighboring countries (14), though they found no strong evidence of ethnicity variability in the expression of hormonal markers in breast tumors. Conversely, a study by Elledge et al., which stratified ER status by age and race in women with breast cancer, indicated that there is no significant difference in ER or PR hormone status by ethnicity in women younger than 35. In contrast, among women older than 35, African-American women were found to have a lower percentage of ER-positive tumors compared to white women (27).
In our study, there was a significant relationship found between tumor grade and its pathological type, mirroring the findings of Li et al. (18). Specifically, women diagnosed with mucinous and papillary carcinoma typically presented with a lower grade compared to those with other subtypes, particularly invasive ductal carcinoma. This study did not find a significant relationship between ER, PR, HER2, and the pathological type of the malignant mass, which stands in contrast to the findings of Li et al. (18). This discrepancy highlights the complexity of breast cancer and the need for further research to fully understand the interactions between molecular markers and pathological types.
Our results indicate that the mean age of patients with infiltrating ductal carcinoma, the most prevalent histological subtype, was 49.4 years. DeSantis et al. noted that luminal A is the most common subtype in postmenopausal white women, accounting for 67% of all breast cancers in this demographic (28). Fatma Khinaifis's cohort study at King Abdul Aziz University Hospital in Saudi Arabia found that over 45% of luminal A, 41% of luminal B, and 41.2% of HER-2 positive cases occurred in the age group over 50 years. Conversely, nearly 70% of triple-negative patients were under 50 years old (20).
Strengths of the study:
- Novelty: This study is the first to investigate the histopathological characteristics of breast cancer in the Kurdish-speaking population in Iran, providing valuable insights into this specific ethnic group.
- Comprehensive analysis: A detailed examination of the histopathological characteristics was conducted alongside the pathological and molecular classification, enhancing the depth of the findings.
Limitations of the study:
- Retrospective nature: The retrospective design limits the ability to follow up with patients to evaluate survival based on various factors such as pathological and molecular types, tumor grade and size, and prognostic biomarkers (ER, PR, HER2, and Ki-67).
- Lack of comprehensive data: The study did not have access to other potentially influential variables such as social class, nutrition, lifestyle, and genetic factors, which could have provided further context to the findings.
- Ethnic comparisons: The absence of comparative data from other ethnic groups within Iran due to the scarcity of comprehensive studies limits the ability to generalize the findings across different demographics within the country.
In summary, while the study contributes significantly to the understanding of breast cancer in the Kurdish population of Iran, the noted limitations underscore the need for prospective studies and broader research to build upon these initial findings.
5.1. Conclusions
Our data revealed that infiltrating ductal and lobular carcinoma are the most prevalent pathological types, and luminal A is the most frequent molecular subtype among Kurdish women in Iran. Among the primary biomarkers of breast cancer, including ER, PR, HER2, and Ki-67, Ki-67 was the only biomarker that showed a significantly different distribution among pathological types. Specifically, a higher percentage of cases with Ki-67 positivity was observed in three types of breast cancer: Infiltrating ductal carcinoma, metaplastic, and papillary carcinoma, compared to the other two types, lobular and mucinous carcinoma.
The findings of this study suggest that a deeper investigation into the survival rates of patients with malignant breast tumors, based on their pathological and molecular subtypes and considering potential prognostic biomarkers, could greatly aid clinicians. Such research would enhance understanding and improve the management of breast cancer in the Kurdish population. This approach could lead to more tailored and effective treatment strategies, ultimately improving patient outcomes in this specific regional context.