1. Background
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer. While L-asparaginase induces complete remission in over 85% of patients, approximately half of the cases relapse with unfavorable outcomes (1). Current therapies have adverse side effects that decrease life expectancy, highlighting the need to reduce cytotoxic effects and explore better treatment regimens (2-4). The Reishi or Lingzhi mushroom is widely used in China and other Asian countries for its pharmacological activity, primarily due to its polysaccharides and triterpenoids (5). Ganoderic Acid A (GAA), isolated from Ganoderma lucidum, exhibits remarkable cytotoxic effects against various cancers. It suppresses cell growth, promotes apoptosis, and can serve as an additional therapeutic intervention for malignancies (6-8).
Autophagy is a crucial cellular pathway that maintains homeostasis and can help cells survive during stress. However, autophagy induction can increase tumor cell survival under adverse conditions, potentially playing a role in leukemia development (9). This study investigated GAA's antineoplastic effects on NALM-6 cells, focusing on changes in autophagy gene expression (MAP1LC3B, BECN1, ATG5, ATG10, RB1CC1, and AMBRA1) before and after treatment with GAA extract of G. lucidum and its relationship with apoptosis induction (10).
2. Objectives
The study aims to provide insight into GAA's potential as a natural antineoplastic agent for ALL treatments, with fewer side effects.
3. Methods
3.1. Study Design
In this case-control study, the NALM-6 B-cell line was obtained from the Iranian Biological Resource Center (IBRC) and cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 100 U/mL penicillin-streptomycin solution. All ingredients and reagents used in cell culture were provided by Sigma-Aldrich (Munich, Germany). Cell viability was ensured using conventional methods and the trypan blue exclusion technique. The GAA extract, purchased from Sigma-Aldrich (St. Louis, MO, USA), was stored at -80°C in a dimethyl sulfoxide (DMSO) stock solution at a concentration of 5 mg/mL.
3.2. MTT Assay
To assess the inhibitory effect of GAA on cell metabolic activity, an MTT assay was conducted. NALM-6 cells were seeded in a 96-well plate and cultured for 24, 48, and 72 hours with different concentrations of GAA (25, 50, 100, 200, and 400 μg/mL). A total of 20 µL of freshly prepared MTT solution was added to each well, and the plate was incubated before measuring the spectrometric absorbance at 570 nm using a BioTek ELx800 microplate photometer (BioTek, Winooski, VT, USA). The IC50, the concentration of medication that inhibited cell growth by 50%, was calculated. Each experiment was performed in triplicate.
3.3. RNA Extraction and Complementary DNA Synthesis
Cellular RNA was extracted from each group using trizol reagent (Thermo Fisher Scientific, USA). The amount and quality of RNA were measured using an RNA spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, USA), and the RNA was then stored at -80°C. Next, 3.7 μL of each RNA sample was reverse-transcribed into complementary DNA (cDNA) using prime script first strand cDNA synthesis kit (Pars Toos, Tehran, Iran) following the manufacturer's instructions, bringing the final volume to 20 μL.
3.4. Quantitative Real-time PCR
Primers for MAP1LC3B, ATG5, ATG10, RB1CC1, and AMBRA1 were designed using AlleleID version 7 and Gene Runner software (Table 1). Quantitative real-time PCR (qRT-PCR) was performed using a QIAGEN real-time PCR cycler (Rotor-Gene, Germany), with ACTB (beta-actin) as the endogenous control to evaluate gene expression.
| Genes | Primer Pair | Product Length (bp) | Product Melt Temperature (°C) |
|---|---|---|---|
| ACT-β | 177 | 90 | |
| Forward | ATCGTGCGTGACATTAAGGAG | ||
| Reverse | GAAGGAAGGCTGGAAGAGTG | ||
| MAP1LC3 | 208 | 85 | |
| Forward | GTGATAATAGAACGATACAAGG | ||
| Reverse | CACTCTCATACACCTCTG | ||
| BECN1 | 154 | 87 | |
| Forward | TGGCACAATCAATAACTTC | ||
| Reverse | GTAAGGAACAAGTCGGTAT | ||
| ATG5 | 152 | 83 | |
| Forward | TTGTCCTTCTGCTATTGAT | ||
| Reverse | GCTGTGGGATGATACTAAT | ||
| ATG10 | 185 | 84 | |
| Forward | TGATTGTGAAGTGATTGAAAC | ||
| Reverse | GTAGCAGTCGCATCTTAT | ||
| RB1CC1 | 99 | 80 | |
| Forward | TGTCGTCTCCTAATCCTAT | ||
| Reverse | GCATTCGTCTACTGTCTAT | ||
| AMBRA1 | 93 | 82 | |
| Forward | CGCTCTACCTTCTTATTGG | ||
| Reverse | AGTCTTCACCTCCGTAATA |
Each real-time PCR reaction contained cDNA, SYBR Green PCR master mix (SYBR premix Ex Taq II, Tli RNaseH Plus, Yektatajhiz, Iran), primers, and nuclease-free water in a final volume of 20 µL, and was run for 45 cycles under specific conditions. The qRT-PCR assay was conducted under the following conditions: Initial denaturation at 95°C for five minutes, followed by 45 cycles of 95°C for five seconds, 59°C for 20 seconds, and 72°C for 30 seconds. Each sample was analyzed in triplicate. The 2-ΔΔCT method was used to analyze fold changes (FC) in gene expression, with each sample run in triplicate. The comparative Ct method (RQ = 2-ΔΔCT) was used to determine the relative expression levels of all genes.
3.5. Flow Cytometry
The percentage of apoptotic cells following GAA treatment was measured using a kit developed by BD Biosciences (San Jose, CA). Briefly, NALM-6 cells were collected 48 hours after treatment with the specified dose of GAA isolated from G. lucidum. The cells were washed twice with phosphate-buffered saline (PBS) before resuspending 5 × 105 cells in 1 mL of 1X annexin V binding buffer. The cells were then incubated in the dark for 20 minutes at room temperature with 5 μL of fluorochrome-conjugated annexin V and 5 μL of propidium iodide (PI) staining solution. After incubation, 400 μL of binding buffer was added to the cell suspension, which was then centrifuged at 400 g for 5 minutes. Cells that were annexin V-positive/PI-negative were considered to be in the early stages of apoptosis, while those that were positive for both annexin V and PI were believed to be in the late stages of apoptosis or necrosis. A FACSCalibur flow cytometer (BD Biosciences, USA) was used to identify the apoptotic population, and the data were analyzed using FlowJo software (TreeStar LLC, USA).
3.6. Statistical Analysis
The results are presented as the median FC in expression from three separate studies. GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA) was used for analysis. One-way ANOVA and Fisher's exact test were conducted to compare different groups, with a significance level set at P < 0.05.
4. Results
4.1. The Significance of the Anti-proliferative Effects of Ganoderic Acid A on Human NALM-6 Cancer Cells
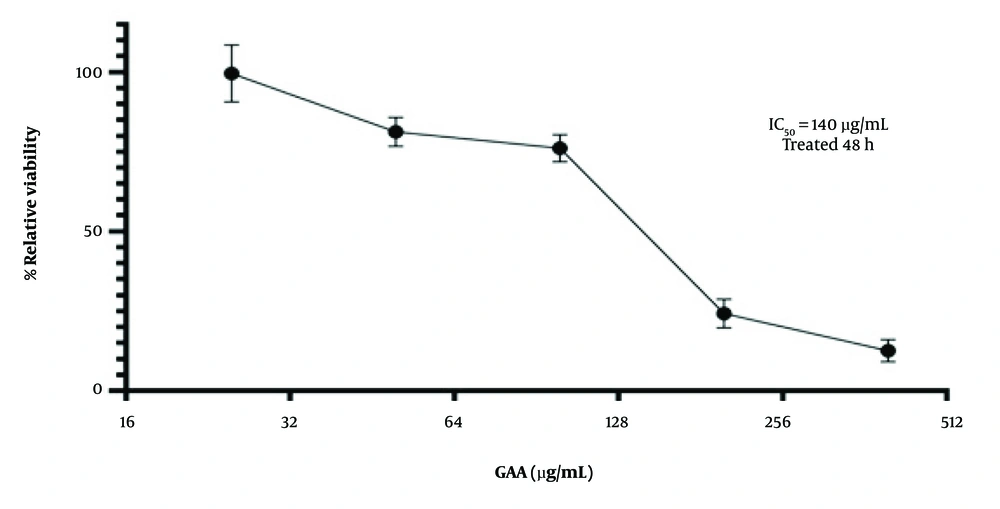
The present study found that GAA significantly reduced the proliferation of NALM-6 cells. An MTT assay was performed to assess cell viability after treatment with different concentrations of GAA (25, 50, 100, 200, and 400 μg/mL) for 24, 48, and 72 hours. The results demonstrated a dose- and time-dependent decrease in cellular viability. The IC50 value was determined to identify the optimal GAA concentration, and the NALM-6 cells exhibited significantly reduced viability in the presence of 140 μg/mL of GAA for 48 hours (P < 0.0001). These findings clearly demonstrate the antiproliferative effects of GAA on human NALM-6 cancer cells (Figure 1). Notably, subsequent tests were conducted using 140 μg/mL of GAA after 48 hours of treatment.
The IC50 of ganoderic acid A (GAA) treatment in the Nalm-6 cells was determined by MTT assay. The Nalm-6 cells (2 × 104 cells/well) were plated into 96-well plates and treated with increasing concentrations (25, 50, 100, 200, and 400 μg/mL) of GAA for 24, 48 and 72 hours. Data are shown as mean ± SD in triplicate. The cells treated without GAA were used as the controls.
4.2. Effect of Ganoderic Acid A Extract on Apoptosis in NALM-6 Cells
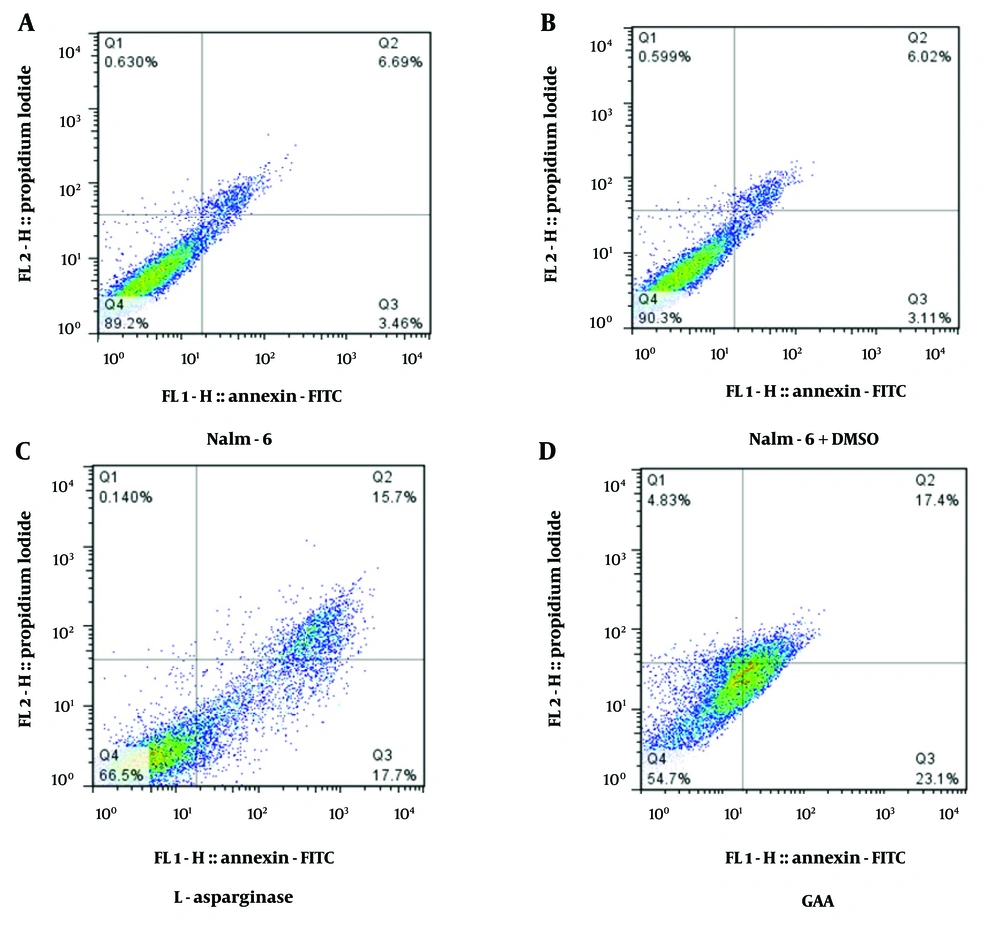
Based on the results presented in Figure 2, the negative control group of NALM-6 cells (without any treatment) had a baseline apoptosis rate of 10.15%. Figure 2B shows NALM-6 cells treated with DMSO to assess any potential side effects of DMSO on NALM-6 cells, and this group had a similar apoptosis rate of 9.13% compared to the negative control group (Figure 2A). Figure 2C shows NALM-6 cells treated with L-asparaginase as the positive control, which had a significantly higher apoptosis rate of 33.4% compared to the negative control and DMSO-treated cells (P < 0.001; Figure 2). In Figure 2D, the NALM-6 cells were treated with GAA at a concentration of 140 μg/mL for 48 hours to evaluate the induction of apoptosis. The findings demonstrated a significantly higher proportion of apoptotic cells in the treated cells compared to the control groups. Specifically, our results indicated 40.5% of early and late apoptosis after 48 hours of treatment (P < 0.0021; Figure 2).
The effects of Ganoderic acid A (GAA) and L-asparaginase on apoptosis in Nalm-6 cells were compared to two control groups: Nalm-6 cells and Nalm-6 cells treated with DMSO. A, Flow cytometry chart showing the distribution of necrotic cells (Q1), late apoptosis (Q2), early apoptosis (Q3), and live cells (Q4); B, the effect of DMSO as a control on Nalm-6 cells; C, results from the induction of apoptosis by L-asparaginase on the Nalm-6 cell line. The cells were exposed to 8 μM of L-asparaginase, and the induction of apoptosis after 48 hours of incubation was compared with untreated cells (P = 0.0112); D, results from the induction of apoptosis by treatment with 140 μg/mL of GAA on the Nalm-6 cell line. The Nalm-6 cells were exposed to the intended concentration of the GAA extract, and the induction of apoptosis after 48 hours of incubation was evaluated (P = 0.0021). The error bars in the diagram represent the standard deviations of two separate tests.
4.3. Comparison of Autophagic Gene Expression in NALM-6 Cells Treated with Ganoderic Acid A Versus L-Asparaginase
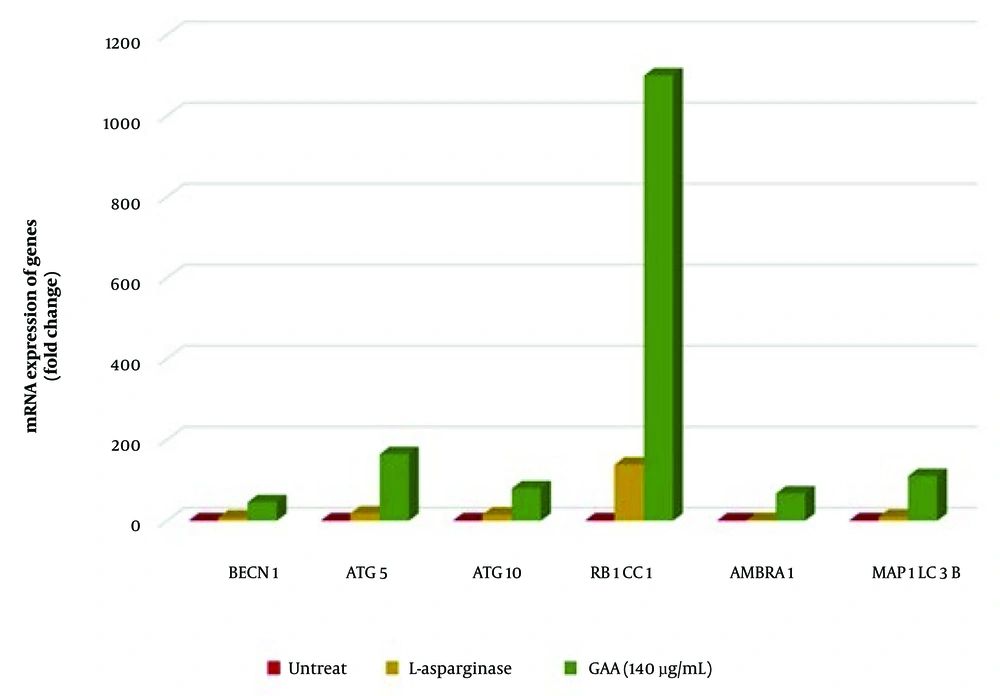
This study reports the first evaluation of GAA's effect on autophagy-related gene expression in NALM-6 cells using qRT-PCR. In Figure 3, we display the effect of 140 μg/mL GAA treatment for 48 hours on the expression of six autophagy-related genes (BECN1, ATG5, ATG10, RB1CC1, AMBRA1, MAP1LC3B) in NALM-6 cells. Our results demonstrate that GAA treatment significantly increased the expression levels of these genes compared to untreated cells. Specifically, MAP1LC3B (P = 0.024; median FC of expression = 111), BECN1 (P = 0.035; FC = 46.2), ATG5 (P = 0.024; FC = 165), ATG10 (P = 0.024; FC = 80.44), RB1CC1 (P = 0.024; FC = 1184), and AMBRA1 (P = 0.024; FC = 67.64) were significantly upregulated (Table 2). Figure 4 illustrates these findings with a heat map.
Quantitative real-time PCR (qRT-PCR) analysis revealed significant differences in the expression MAP1LC3B, BECN1, ATG5, ATG10, RB1CC1, and AMBRA1 genes in Nalm6 cells treated with Ganoderic acid A (GAA) and L-ASP compared to untreated cells. All genes showed upregulation (P < 0.0001) in response to GAA treatment compared to untreated cells. Similarly, the expression levels of all genes were increased in response to L-ASP treatment compared to untreated cells.
| Genes | Treatment with | FC |
|---|---|---|
| Becline | GAA | 46.2 |
| L-asparaginase | 8.33 | |
| ATG5 | GAA | 165 |
| L-asparaginase | 18.37 | |
| ATG10 | GAA | 80.44 |
| L-asparaginase | 15.13 | |
| Fib200 | GAA | 1184 |
| L-asparaginase | 139 | |
| AMBRA | GAA | 67.64 |
| L-asparaginase | 2.158 | |
| LC3 | GAA | 111 |
| L-asparaginase | 10.26 |
Abbreviations: GAA, Ganoderic acid A; FC, fold change.
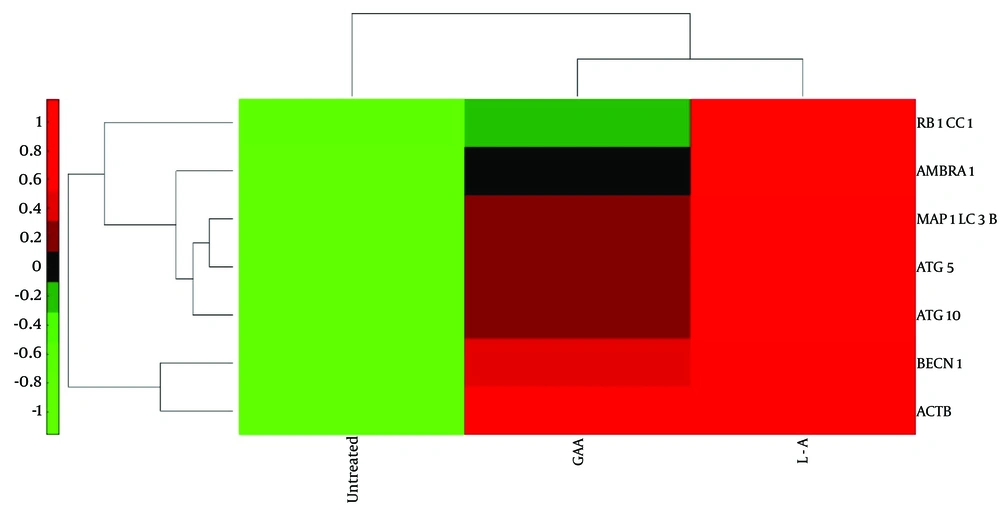
Heat map was generated using hierarchical clustering to visualize the expression MAP1LC3B, BECN1, ATG5, ATG10, RB1CC1, and AMBRA1 in Nalm6 cells treated with Ganoderic acid A (GAA), L-ASP, and untreated cells. The red color indicates lower expression levels compared to the reference channel, while the green color indicates higher expression levels. The heat map showed that GAA treatment resulted in increased expression levels of all six genes, as indicated by the abundance of green-colored cells. In contrast, untreated cells and those treated with L-ASP showed lower expression levels of these genes, as indicated by the abundance of red-colored cells.
5. Discussion
Ganoderic acid A is a triterpenoid from G. lucidum used in Chinese herbal medicine to treat various diseases, including cancer (11). It has significant anticancer properties against various human cancer cells in vitro, but its molecular mechanism in NALM-6 B-cell leukemia is unknown (12). Apoptosis is a cell death process that can prevent the proliferation of malignant tumors (13, 14). Previous studies have examined the effect of G. lucidum on apoptosis in ALL cell lines, and in this study, GAA was selected as a potential inhibitor of leukemic cell proliferation (15, 16).
Results showed that GAA induced apoptosis in NALM-6 cells, with a rate of 40.3% compared to 33.4% in L-asparaginase-treated cells (Figure 2C). This suggests that GAA has the potential to reduce cell proliferation in NALM-6 cells by inducing apoptosis, making it a promising natural cancer treatment. Many investigations have shown that autophagy has a dual impact on cancer, either inhibiting tumor growth or halting cancer advancement, which appears to rely on unidentified features of diverse cancer forms. This analysis outlines the main objectives related to autophagy and the evolution of malignancy. Furthermore, the conflicting roles of autophagy in cancer as a suppressor of tumors or as a promoter of cancer, along with potential treatment options using modulators of autophagy or a combination of therapies with anticancer medications, have been explored (17).
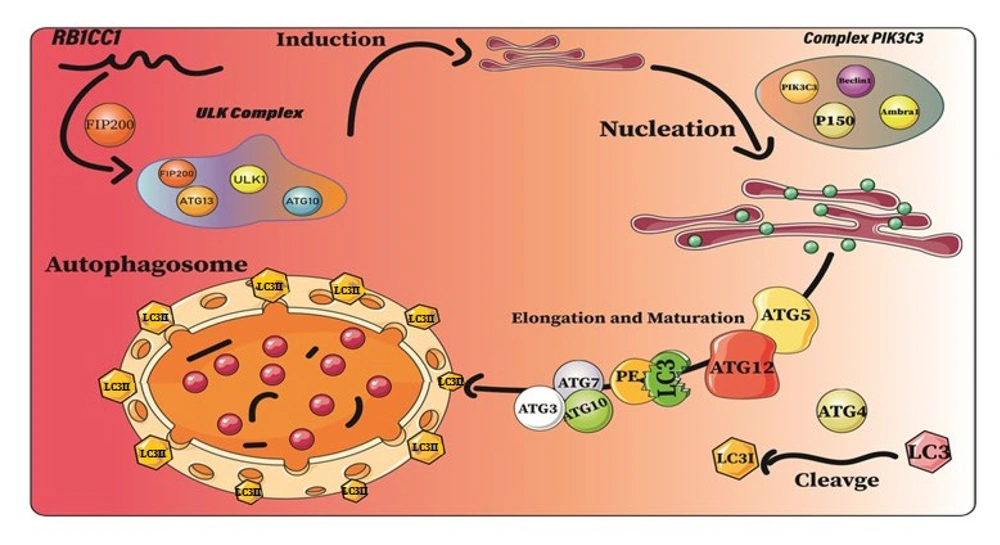
Autophagy plays a role in leukemia initiation and progression, as leukemic cells use it for metabolic demands for proliferation and survival (Figure 5). Activating autophagy can also promote leukemogenesis and drug resistance, making it a potential therapeutic target for leukemia treatment (Figure 4) (18, 19).
The current study found that GAA treatment decreased cell proliferation, induced apoptosis and autophagy, and was cytotoxic to NALM-6 cells in vitro. These results are consistent with previous studies that demonstrated the cytotoxic effects of GAA on various cancer cell lines, including leukemia (20). These findings suggest that GAA may have potential as a natural agent for inhibiting leukemic cell proliferation, promoting apoptosis and autophagy, and developing novel therapeutic strategies for leukemia.
In this study, we used qRT-PCR to assess the expression of autophagic genes, including MAP1LC3B, BECN1, ATG5, ATG10, RB1CC1, and AMBRA1, in NALM-6 cells before and after treatment with GAA extract, as shown in Figure 3. Our results showed that GAA treatment significantly increased the expression levels of these autophagic genes compared to the control groups, which included untreated cells and cells treated with L-asparaginase (Figure 3). These findings are consistent with previous studies by Reis et al., who studied breast cancer cells and found that G. lucidum induced autophagy by increasing the expression of ULK1, Atg5, Atg12, Beclin1, and LC3, which reduced cell growth. Additionally, Li et al. conducted a study on colorectal cancer cells, including SW620 cells, and demonstrated that G. lucidum reduced cancer cell growth, decreased Bcl-2 expression, increased apoptosis, and induced autophagy (18, 21-23). Furthermore, Reis et al. investigated the effect of methanolic extract of G. lucidum on gastric adenocarcinoma cancer cells and found that it increased LC3-II levels, decreased P62 levels, and induced autophagy (21). These studies, along with our findings, suggest that G. lucidum and its components, such as GAA, have the potential to induce autophagy and reduce cell growth.
In a study on acute myeloid leukemia patients, it was found that the expression of the *BECN1* gene was higher in AML patients than in controls, and low expression may be associated with complete recovery. This difference may be due to the dual role of autophagy in tumorigenesis and the pathogenesis of ALL, as well as differences in autophagic activity between populations and ALL subgroups (21). Surprisingly, no previous study has investigated the effect of GAA on the expression of autophagy genes in leukemia cells. This study is the first to evaluate this effect, showing that GAA treatment increased the expression levels of autophagy genes in NALM-6 cells, which may contribute to the therapeutic effect of GAA and increase cell death.
The PI3K/AKT/mTOR pathway regulates many biological processes, including cell proliferation, differentiation, survival, apoptosis, autophagy, tumorigenesis, and angiogenesis. The GAA has been shown to strongly inhibit this pathway in human glioblastoma cells in previous studies (23). The PI3K/AKT/mTOR signaling network plays a role in the occurrence and progression of tumors, including leukemia (24). Inactivation of at least one component of this pathway has been associated with tumorigenesis. mTOR is a crucial regulator of autophagy and inhibits this pathway. Additionally, some signaling pathways, including the class I PI3K/Akt pathway, can inhibit both autophagy and apoptosis pathways. Autophagy can either promote cell survival by inhibiting apoptosis or activate apoptosis, making it an antagonist of apoptosis. Targeting the PI3K/AKT/mTOR pathway may be a potential therapeutic strategy for cancer treatment, including leukemia (25).
The GAA treatment inhibits this pathway, contributing to its antitumor effects, including apoptosis and autophagy induction, and may have the potential for developing cancer therapeutics. The GAA is hypothesized to increase the expression of autophagy genes, particularly RB1CC1, and inactivate the PI3K/AKT/mTOR signaling pathway, leading to apoptosis in NALM-6 cells. Ganoderic acid A has the potential to inhibit cancer cell growth and expansion and increase the expression of autophagy genes. However, more research is needed to determine the exact mechanisms of GAA as an anticancer agent for leukemia treatment (26).
The use of GAA or other active compounds derived from medicinal plants, alone or in combination with chemotherapy drugs, may offer a promising approach for achieving better treatment outcomes with fewer side effects, particularly for ALL, a common and fatal disease among children. In conclusion, the study found that GAA has a potent cytotoxic effect on NALM-6 cells by inducing apoptosis and increasing the expression levels of autophagic genes, including MAP1LC3B, BECN1, ATG5, ATG10, RB1CC1, and AMBRA1. These results suggest that GAA may be a natural agent for the treatment of acute B lymphoblastic leukemia with potentially fewer side effects. Our study sheds new light on the potential mechanisms underlying GAA's therapeutic effects in leukemia.