1. Background
Type 2 diabetes mellitus (T2DM) is a metabolic disease with complex causes, manifestations, and complications (1). The disease is characterized by insulin resistance and the destruction of β-cells in connection with inflammation (2). The genus Ferula is an herbaceous plant belonging to the Apiaceae family. It contains more than 170 species distributed in Central Asia, Eastern Iran, and Afghanistan. One of the Persian names for this plant is Anghuzeh (3).
Animal studies have shown that Ferula assa-foetida extract has protective therapeutic effects in diabetes by lowering plasma glucose levels and increasing serum insulin levels in diabetic rats (4). In addition, oral administration of assa-foetida at doses of 1.25% and 2.5% w/w significantly restored the level of the depleted antioxidant system in Sprague-Dawley rats and significantly inhibited lipid peroxidation in the liver of rats (5). Administration of F. assa-foetida resin has also shown effects in preventing hepatic steatosis in type 2 diabetic rats, associated with decreased serum leptin levels (6).
From the perspective of the mechanism, the anti-diabetic effect of F. assa-foetida can be mediated by its protective role in pancreatic β-cell membrane integrity (4) and its antioxidant activities due to its high content of total phenols and flavonoids, including phenolic acid gallic and flavonoids such as quercetin. Additionally, the extract of this plant has shown different levels of antioxidant activity in the laboratory, including the ability to chelate with iron ions (Fe2+) and eliminate diphenyl-2-picryl hydrazyl (DPPH) and nitric oxide free radicals (7). However, more detailed studies of assa-foetida properties require clinical trials (3).
2. Objectives
Due to the lack of clinical trial studies examining the effect of F. assa-foetida in diabetic patients, we aimed to evaluate the anti-hyperglycemic, anti-hyperlipidemic, anti-inflammatory, and antioxidant effects of the herbal drug F. assa-foetida (assa-foetida) in patients with type 2 diabetes.
3. Methods
3.1. Subjects, Study Design, and Protocol
This study was designed as a randomized, double-blinded, placebo-controlled trial conducted at Ayatollah Taleghani Hospital, Abadan University of Medical Sciences, Abadan, Iran. The study adhered strictly to the clinical research guidelines of the Government of Iran, following the protocol approved by the registered Ethical Committee (ethic code: IR.ABADANUMS.REC.1398.024) dated 2019-06-16. Additionally, this clinical trial has been approved by the Iranian Registry of Clinical Trials (IRCT registration number: IRCT20190525043704N1) dated 2019-09-23.
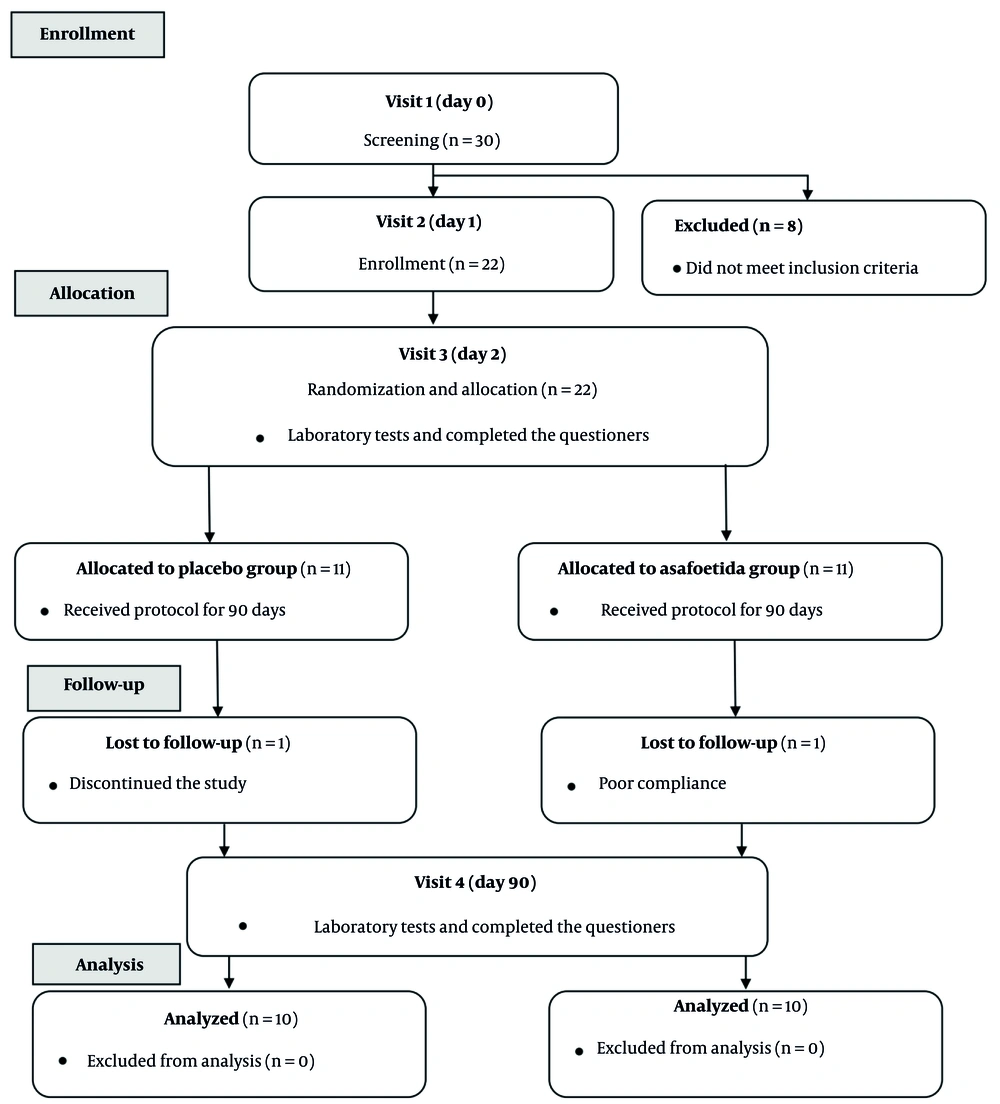
The subjects were selected from patients who visited the doctor for medical advice about type 2 diabetes. Those subjects who fulfilled the seven screening criteria under the widely used Wilson-Jugner criteria were enrolled in the study with written informed consent (8, 9). The protocol applied in the present study is illustrated in Figure 1. The total study period was 90 days.
During the first visit on day 0, an internal specialist physician screened the adult patients with T2DM to participate in the study, taking into account the inclusion and exclusion criteria. During the second visit (day 1), the basic characteristics of the patients and the anthropometric measurements, including height (H, cm), weight (W, kg), waist circumference (WC, cm), and hip circumference (HC, cm), were obtained using a stadiometer, a digital scale (Omron, BF511, OMRON HEALTHCARE Co., Ltd. Kyoto, Japan), and a tape measure, respectively. In addition, Body Mass Index (BMI; kg/m2) was calculated by dividing weight by height squared, and WHR was obtained by dividing WC by HC. Moreover, systolic and diastolic blood pressures (mmHg) were evaluated using an automatic blood pressure monitor (Model M6 AC, OMRON HEALTHCARE Co., Ltd. Kyoto, Japan).
On the third visit (day 2), patients were divided into assa-foetida and Placebo groups. The intervention group received polyethylene containers containing 180 capsules labeled assa-foetida at a dose of 250 mg twice daily, and the control group received polyethylene containers containing 180 starch capsules at a dose of 250 mg twice daily as a placebo. Patients were instructed to take one capsule before breakfast and one capsule before dinner (250 mg twice daily) (10). At the end of the study, the compliance rate of prescriptions was assessed using the pill-counting strategy.
All subjects in both the intervention and control groups continued receiving their current hypoglycemic drugs, glibenclamide, and metformin, during the study. During the third (day 2) and the fourth visits (day 90), 8 cc of blood was drawn when the patient had been fasting for at least 8 and at most 16 hours for biochemical analysis.
3.2. Inclusion and Exclusion Criteria
Our inclusion criteria were age 25 - 70 years, male and female patients, fulfilling diagnostic criteria for T2DM (11), and willing to provide written informed consent.
Exclusion criteria included the consumption of warfarin and other Coumarin substitutes by the patient; use of blood glucose-boosting drugs such as antidepressants (Tricyclics), beta-adrenergic blockers, corticosteroids, diazoxide, diuretics, epinephrine, estrogen, glucagon, isoniazid, lithium, phenothiazines, phenytoin, salicylates, and triamterene; use of blood glucose-lowering drugs including acetaminophen, alcohol, anabolic steroids, gemfibrozil, monoamine oxidase inhibitors, propranolol, tolazamide, and tolbutamide; history of uncontrolled hypertension, congestive heart failure, and other cardiovascular diseases, liver, kidney, or any metabolic and clinical disorders other than T2DM; pregnancy and lactation in women; psychological diseases; being treated with insulin; smoking during the test period (as it raises blood glucose levels); having sickle cell anemia, thalassemia, and chronic renal failure that affect hemoglobin A1c levels; and any circumstances that, in the opinion of the researcher, do not support the participation of patients in the study (10).
3.3. Randomization and Blinding
3.3.1. Creating a Random Sequence
In this study, random sampling was performed using a random numbers table to create a random sequence based on the method described in the Vaidyanathan study (12).
3.3.2. Allocation Concealment
Opaque, sealed, and waxed envelopes were used sequentially to hide random allocation (numbered, sealed, opaque envelopes). In this method, after creating a random sequence, based on the research sample size, several envelopes with aluminum wrapping (to obscure the contents of the envelopes) were prepared. Each of the created random sequences was recorded on a card, and the cards were placed in the envelopes to preserve the random sequence. The outside of the envelopes was numbered in the same order. Finally, the envelopes were sealed and placed inside a box. The envelopes were opened in order, and the assigned group of that participant was revealed. One of the researchers prepared supplements or capsules with the same color and shape (placebo) and placed them in the envelope according to the allocation order. These supplements could not be distinguished because they contained cans with the same color, shape, and label.
3.3.3 Implementing the Random Allocation Process
In this study, the researcher who created the random sequence was separated from other researchers during the stage of registering the names or allocation of participants to reduce possible bias.
3.3.4 Blinding
This study was conducted in a double-blind manner. In this way, both the participants and the researchers were unaware of the allocation of the study groups. The placebos were completely similar to the F. assa-foetida supplement in terms of color, appearance of the cans, gelatin capsules, smell, and the method of use that was included on the placebo cans so that study participants were blind about the allocated group.
3.4. Primary Outcome
The primary outcomes included changes in: (1) Glucose metabolism, which was measured by fasting, two-hour postprandial, and HbA1c; (2) hepatic function, which was evaluated by liver function tests; (3) oxidative stress, which was assessed by antioxidant enzyme activity; (4) complications of chronic hyperglycemia, which were evaluated by advanced glycation end product (AGE) formation, from baseline to 90 days of follow-up in the assa-foetida group compared with placebo.
3.5. Secondary Outcome
The secondary outcome was determined based on the effect of assa-foetida compared with placebo on the comorbidities of diabetes [anthropometric measurements, blood lipid concentrations, blood pressure, leptin, and high-sensitive C-reactive protein (hs-CRP) concentrations] at baseline and 90 days after assa-foetida administration.
3.6. Preparation and Formulation of the Assa-foetida and Placebo
Iranian F. assa-foetida was employed for the preparation of assa-foetida capsules. The root and stem of F. assa-foetida L. were purchased from a local medicinal plant market in Kerman, Iran. The combination of drug formulations used in the same study (13) (plant roots and seeds) was used to prepare assa-foetida capsules in the Department of Pharmacology, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences with Herbarium Voucher Number 981, and then they were formed into capsules in a dose of 250 mg.
3.7. Safety Assessment of Ferula assa-foetida and Side Effects
Ferula assa-foetida drug toxicity at a dose of 250 mg twice daily has been confirmed based on a previous study. Moreover, the use of this medicinal plant in humans has not shown any side effects (13).
3.8. Biochemical Assessment
The calorimetric method using a spectrophotometric device was used to measure glycemic and lipid profiles, and hepatic enzyme activity. The activity of catalase, serum levels of hs-CRP, and MDA was measured by the calorimetric method with an ELISA reader. Measurement of AGEs and leptin serum levels was performed by the ELISA method with an ELISA reader.
3.9. Dietary Assessment
To evaluate dietary intake, a Qualitative Food Frequency Questionnaire (FFQ) was used (14). The FFQ was completed for all patients during the second visit (day 1) and the fourth visit (day 90). Based on previous studies, the food items on the FFQ were grouped according to their nutrient contents (14, 15). Therefore, seventeen food groups were derived as follows: (1) Whole grains, (2) refined grains, (3) potatoes, (4) dairy products, (5) vegetables, (6) fruits, (7) legumes, (8) meats, (9) nuts and seeds, (10) solid fat, (11) liquid oil, (12) tea and coffee, (13) salty snacks, (14) simple sugars, (15) honey and jams, (16) soft drinks, and (17) desserts and snacks. Possible response categories were “daily”, “weekly”, “monthly”, “annually”, and “never”. The responses were scored according to a researcher-based scoring system as follows: “daily: 4”, “weekly: 3”, “monthly: 2”, “annually: 1”, and “never: 0”. Responses were added to determine the score of consumption frequency of each food group.
3.10. Assessment of Physical Activity
An assessment of physical activity (PA) was performed using a short version of the International Physical Activity Questionnaire (IPAQ) in the ‘‘last 7 days’’ during the second visit (day 1), and the interpretation of the results was based on a procedure explained in the Mynarski et al. study (16).
3.11. Sample Size Calculation
We used a similar study for the calculation of sample size (10). The number of samples in each group was calculated taking into account δ1 = 2.50, μ1 = 89.92, δ2 = 2.57, μ2 = 95.11, α = 0.05, and power 90%, resulting in 6 patients. The sample size calculation was performed based on the formula as below (17):
We have decided to include a total of 20 patients, with 10 patients in each group, to ensure statistical robustness in our analysis.
3.12. Statistical Analyses
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA) version 26. Data normality was checked by the Shapiro–Wilk test. For normally distributed data, a paired sample t-test was used for within-group comparison of a specific parameter, and between-group comparison of that parameter was performed using an independent t-test. For non-normally distributed data, the Wilcoxon Signed Ranks test was applied for within-group comparison of a specific parameter, and the Mann-Whitney U test was used for between-group comparison of that parameter. Moreover, differences in the frequency of variables were compared using the chi-square (χ2) test. The results are displayed as mean and standard deviation (mean ± SD) for normal distribution parameters and median (IQR) for non-normal distribution parameters.
4. Results
4.1. Baseline Characteristics of Participants
The baseline characteristics are provided in Table 1. No significant differences were found between placebo and assa-foetida-treated groups in demographic characteristics at baseline.
a Values are expressed as mean ± standard deviation (SD) for quantitative and No. (%) for qualitative data.
b Independent samples t-test was applied to analyze quantitative and qualitative data, respectively.
c Chi-square test was applied to analyze quantitative and qualitative data, respectively.
4.2. Follow-up and Adherence
There were no significant group differences in adherence to the assigned treatment, as determined by pill counts. In the assa-foetida group, 5 (50%) of those with 90 days’ data had ≥80% compliance compared with 5 (71.4%) in the placebo group (P = 0.515).
4.3. Effect of Assa-foetida on Physical Activity, Anthropometric, and Clinical Characteristics
4.3.1. Physical Activity Parameters
The present study employed an IPAQ Scale to compare weekly PA measurements in the assa-foetida and placebo groups at baseline and the end of the study. Between-group comparison using the Mann-Whitney U test showed no significant difference between baseline values of the placebo and assa-foetida-treated groups for weekly PA of low intensity. However, declared weekly PA was significantly higher among patients receiving assa-foetida compared with the placebo-treated group at baseline [between-group mean difference of weekly PA of low intensity: -2715.08 METmin/week; 95% CI: -5758.58, 328.43, P = 0.077; between-group mean difference of declared weekly PA: -3540.06 METmin/week, 95% CI: -6714.47, -365.64; P = 0.031]. However, there was no significant difference at the end of study values when compared to the placebo.
4.3.2. Anthropometric and Clinical Characteristics
No significant differences were observed between the groups in terms of weight or clinical characteristics at baseline or study end, as determined by the paired sample t-test/Wilcoxon Signed Ranks test (Table 2).
| Parameters | Placebo | Assa-foetida | P-Value b |
|---|---|---|---|
| Weight (kg) | |||
| Baseline | 78.3 ± 11.1 | 79.7 ± 11.1 | 0.772 |
| Day 90 | 80.2 ± 12.5 | 79.8 ± 11.2 | 0.953 |
| P-value c | 0.731 | 0.701 | - |
| BMI (Kg/m2) | |||
| Baseline | 30.9 ± 4.8 | 29.8 ± 3 | 0.55 |
| Day 90 | 30.4 ± 4.9 | 29.7 ± 2.8 | 0.732 |
| P-value c | 0.908 | 0.553 | - |
| WC (cm) | |||
| Baseline | 105.6 ± 8.4 | 104.6 ± 8.3 | 0.794 |
| Day 90 | 104.6 ± 10.3 | 104.9 ± 10.4 | 0.951 |
| P-value c | 0.467 | 0.952 | - |
| WHR | |||
| Baseline | 0.89 ± 0.3 | 0.97 ± 0.04 | 0.408 |
| Day 90 | 0.97 ± 0.04 | 0.99 ± 0.04 | 0.337 |
| P-value c | 0.29 | 0.354 | - |
| Systolic BP (mmHg) | |||
| Baseline | 147.1 ± 24.8 | 119.4 ± 42.6 | 0.092 |
| Day 90 | 142 ± 27.8 | 125.7 ± 14.1 | 0.154 |
| P-value c | 0.798 | 0.401 | - |
| Diastolic BP (mmHg) | |||
| Baseline | 90.1 ± 9.4 | 77.3 ± 25.6 | 0.156 |
| Day 90 | 89.2 ± 12.3 | 86.9 ± 10.8 | 0.711 |
| P-value c | 0.949 | 0.297 | - |
| PA1 d (METmin/wk) e | |||
| Baseline | 0 (0.6) | 0 (10.9) | 0.315 |
| Day 90 | 0 (39) | 0 (364) | 0.607 |
| P-value c | 0.285 | 0.575 | - |
| PA2 f (METmin/wk) e | |||
| Baseline | 32 (141.8) | 60 (658.5) | 0.28 |
| Day 90 | 40.2 (597.2) | 72 (360) | 0.864 |
| P-value c | 0.138 | 0.889 | - |
| PA3 g (METmin/wk) e | |||
| Baseline | 74.3 (1454.1) | 1386 (4393.1) | 0.011 |
| Day 90 | 635.3 (1163.3) | 693 (3729) | 0.529 |
| P-value c | 0.463 | 0.173 | - |
| DPA c (METmin/wk) e | |||
| Baseline | 238.6 (1578.6) | 3017.8 (5915.1) | 0.011 |
| Day 90 | 695.3 (1619.4) | 1632 (5030.5) | 0.456 |
| P-value c | 0.6 | 0.214 | - |
Abbreviations: BMI, Body Mass Index; WC, waist circumference; WHR, waist to hip ratio; PA, physical activity.
a Values are expressed as mean ± standard deviation (SD) for normal and median (IQR) for non-normal distribution.
b Between-group comparisons were applied based on the independent sample t-test/Mann-Whitney U test.
c Within-group comparisons were applied based on the paired sample t-test/Wilcoxon Signed Ranks test.
d Weekly PA of high intensity.
e Non-parametric tests were applied to analyze data.
f Weekly PA of moderate intensity.
g Weekly PA of low intensity.
4.4. Effect of Assa-foetida on Glycemic, Lipidemic, Hepatic, Inflammatory, and Antioxidant Parameters
4.4.1. Inflammatory Markers (ESR and High-Sensitive C-Reactive Protein)
Assa-foetida increased ESR levels at the end of the study compared to baseline. As Table 3 shows, a within-group comparison using the Wilcoxon Signed Ranks test displayed a significant increase in the end-of-study values of ESR 1st h (mm/h) compared to the baseline in the assa-foetida treated group [within-group mean difference of ESR 1st h (mm/h): -4.8 mm/h, 95% CI: -8.7, -0.84; P = 0.017]. However, this increase was within the normal range (< 20 mm/h). In addition, the Mann-Whitney U test showed that patients in the placebo-treated group had higher ESR 1st h (mm/h) values at baseline compared to the assa-foetida group.
| Parameters | Baseline | Day 90 | P-Value b |
|---|---|---|---|
| ESR 1st h (mm/h) | |||
| Placebo | 29 (27.3) | 19 (23.5) | 0.206 |
| Assa-foetida | 10.5 (11.3) | 12 (8) | 0.017 |
| P-value c | 0.019 | 0.37 | - |
| FBG (mg/dL) | |||
| Placebo | 148.5 ± 65.2 | 146.5 ± 67.1 | 0.732 |
| Assa-foetida | 148.7 ± 43.2 | 163.7 ± 49.9 | 0.316 |
| P-value c | 0.994 | 0.555 | - |
| Two hrspp (mg/dL) | |||
| Placebo | 247.7 ± 115.2 | 234.8 ± 96.2 | 0.996 |
| Assa-foetida | 219.9 ± 73.5 | 241.4 ± 61.6 | 0.242 |
| P-value c | 0.528 | 0.865 | - |
| Hemoglobin A1C (%) | |||
| Placebo | 8.2 ± 1.2 | 8 ± 1.6 | 1.00 |
| Assa-foetida | 8.5 ± 1 | 8 ± 1.6 | 0.184 |
| P-value c | 0.554 | 0.943 | - |
| Cholesterol (mg/dL) | |||
| Placebo | 163 ± 31.1 | 163.3 ± 47.3 | 0.754 |
| Assa-foetida | 141.3 ± 45.8 | 129.4 ± 37.2 | 0.205 |
| P-value c | 0.231 | 0.12 | - |
| Triglycerides (mg/dL) | |||
| Placebo | 155 (142.3) | 169.5 (122.5) | 0.674 |
| Assa-foetida | 109.5 (98.5) | 151 (162) | 0.066 |
| P-value c | 0.075 | 0.673 | - |
| HDL-C (mg/dL) | |||
| Placebo | 40.3 ± 10.5 | 59.4 ± 46.4 | 0.235 |
| Assa-foetida | 40 ± 10.03 | 32.3 ± 6.9 | 0.046 |
| P-value c | 0.949 | 0.104 | - |
| LDL (mg/dL) | |||
| Placebo | 86 ± 25.1 | 86.4 ± 40 | 0.654 |
| Assa-foetida | 76.1 ± 31.1 | 72.3 ± 35.7 | 0.965 |
| P-value c | 0.444 | 0.456 | - |
| LDL-C/HDL-C | |||
| Placebo | 2 (1.8) | 2.3 (2.4) | 0.888 |
| Assa-foetida | 2 (1.3) | 1.6 (1.8) | 0.213 |
| P-value c | 0.436 | 0.888 | - |
| Cholesterol/HDL-C | |||
| Placebo | 3.9 (1.6) | 4 (3.1) | 0.833 |
| Assa-foetida | 3.6 (1.6) | 3.7 (2.1) | 0.137 |
| P-value c | 0.393 | 0.963 | - |
| SGOT (IU/L) | |||
| Placebo | 21.4 ± 7.02 | 17.8 ± 5.03 | 0.363 |
| Assa-foetida | 26.8 ± 9.4 | 21.2 ± 5 | 0.084 |
| P-value c | 0.163 | 0.175 | - |
| SGPT (IU/L) | |||
| Placebo | 15 (14) | 16.5 (7.5) | 0.462 |
| Assa-foetida | 21 (24) | 25 (12) | 0.514 |
| P-value c | 0.063 | 0.036 | - |
| hs-CRP (mg/L) | |||
| Placebo | 4.3 ± 2.3 | 2.4 ± 1.8 | 0.015 |
| Assa-foetida | 2.9 ± 1.9 | 2.9 ± 2.4 | 0.933 |
| P-value c | 0.166 | 0.667 | - |
| AGEs | |||
| Placebo | 169.5 (42.7) | 163.4 (49.1) | 0.612 |
| Assa-foetida | 163.4 (16.8) | 164.4 (26) | 0.066 |
| P-value c | 0.541 | 0.743 | - |
| Catalase | |||
| Placebo | 7.5 ± 2.9 | 9.7 ± 8.1 | 0.44 |
| Assa-foetida | 11.3 ± 4.8 | 14.4 ± 9 | 0.37 |
| P-value c | 0.07 | 0.278 | - |
| Leptin | |||
| Placebo | 26.7 (38.7) | 22 (52.8) | 0.310 |
| Assa-foetida | 12.2 (21.1) | 12.6 (28.2) | 0.779 |
| P-value c | 0.541 | 0.743 | - |
| MDA | |||
| Placebo | 86.3 (138.8) | 33.1 (30.3) | 0.237 |
| Assa-foetida | 41 (50.6) | 29.8 (11) | 0.173 |
| P-value c | 0.277 | 0.815 | - |
Abbreviations: SGPT, serum glutamic pyruvic transaminase; hs-CRP, high-sensitive C-reactive protein; AGEs, advanced glycation end products.
a Values are expressed as mean ± standard deviation (SD) for normal and median (IQR) for non-normal distribution.
b Within-group comparison was applied based on the paired sample t-test/Wilcoxon Signed Ranks test.
c Between-group comparison was applied based on the independent sample t-test/Mann-Whitney U test.
In addition, lower hs-CRP levels were found in the placebo group compared to baseline. A within-group comparison using the paired sample t-test showed a lower hs-CRP value in placebo-treated groups at the end of the study compared to baseline [within-group mean difference of hs-CRP (mg/L): 1.9, 95% CI: 0.5, 3.3; P = 0.015].
4.4.2. Lipid Profile (HDL-C)
A decrease in HDL-C levels was observed in the assa-foetida group by the study’s end based on the paired sample t-test [within-group mean difference of HDL-C (mg/dL): 7.7, 95% CI: 0.2, 14.2; P = 0.046].
4.4.3. Liver Enzyme (Serum Glutamic Pyruvic Transaminase)
Furthermore, a between-group comparison using the Mann-Whitney U test revealed that patients in the assa-foetida-treated group showed higher serum glutamic pyruvic transaminase (SGPT) compared to the placebo-treated group at the end of the study [between-group mean difference of SGPT (IU/L): -7.71 IU/L, 95% CI: -13.92, -1.49; P = 0.036]. However, this increase was within the normal range (5 - 40 IU/L).
4.4.4. Glucose Metabolism
There was no significant difference in the glycemic profile and antioxidant parameters between the assa-foetida and placebo groups at baseline and the end of the study.
4.5. Dietary Assessment of Placebo and Assa-foetida Treated Groups at Baseline and After 90-Day Follow-up
Between-group comparison based on an independent sample t-test indicated that the mean score of taking nuts and seeds (between-group mean difference of taking nuts and seeds: 4.3, 95% CI: -0.4, 8.3; P = 0.033) and desserts and snacks (between-group mean difference of taking snacks and desserts: 9.1, 95% CI: 1.7, 16.6; P = 0.021) food groups reported on FFQs were significantly lower in the assa-foetida group than in the placebo group on day 90 of the study.
Moreover, within-group comparison based on the paired sample t-test/Wilcoxon Signed Ranks test revealed that intakes from whole grains (within-group mean difference of taking whole grains: 12.9, 95% CI: 7.1, 18.6; P = 0.018), refined grains (within-group mean difference of taking refined grains: -7, 95% CI: -13.3, -0.7; P = 0.034), and potatoes (within-group mean difference of taking potatoes: -4, 95% CI: -7.2, -0.8; P = 0.028) food groups were increased significantly on day 90 compared to the baseline among patients taking assa-foetida. However, patients taking assa-foetida consumed more nuts and seeds at baseline compared to day 90 (within-group mean difference of taking nuts and seeds: 2.2, 95% CI: 0.2, 4.2; P = 0.041) on day 90 compared to the baseline.
At the same time, the patients in the placebo group consumed more refined grains (within-group mean difference of taking refined grains: -6.3, 95% CI: -10.6, -2.1; P = 0.012) and legumes (within-group mean difference of taking legumes: -4.7, 95% CI: -8.8, -0.49; P = 0.035) (Table 4).
| Parameters | Placebo | Assa-foetida | P-Value b |
|---|---|---|---|
| Whole grains | |||
| Baseline | 7.5 (6) | 11 (5) | 0.145 |
| Day 90 | 27.5 (10) | 23 (3) | 0.073 |
| P-value d | 0.068 | 0.018 | - |
| Refined grains | |||
| Baseline | 15 (4.25) | 16 (9.25) | 0.963 |
| Day 90 | 22 (5.5) | 20.5 (6) | 0.762 |
| P-value d | 0.012 | 0.034 | - |
| Potatoes c | |||
| Baseline | 5 (4.5) | 2 (3.75) | 0.133 |
| Day 90 | 5.5 (4.25) | 6 (4) | 0.864 |
| P-value d | 0.891 | 0.028 | - |
| Vegetables | |||
| Baseline | 69 (12.5) | 71 (17.5) | 0.606 |
| Day 90 | 64 (8.25) | 64 (15.5) | 0.973 |
| P-value d | 0.073 | 0.288 | - |
| Fruits | |||
| Baseline | 144 (39) | 133 (33.25) | 0.328 |
| Day 90 | 147.5 (55) | 116 (64) | 0.341 |
| P-value d | 0.362 | 0.747 | - |
| Legumes | |||
| Baseline | 14.5 (2.5) | 12 (5) | 0.501 |
| Day 90 | 20 (5.25) | 18 (9) | 0.957 |
| P-value d | 0.035 | 0.152 | - |
| Dairy products | |||
| Baseline | 55.5 (5.5) | 60 (17.5) | 0.94 |
| Day 90 | 54.5 (15) | 49.5 (17.25) | 0.564 |
| P-value d | 0.643 | 0.227 | - |
| Meats c | |||
| Baseline | 74.5 (9.75) | 66 (14.5) | 0.139 |
| Day 90 | 71 (18) | 55.5 (6.25) | 0.052 |
| P-value d | 0.713 | 0.104 | - |
| Nuts and seeds | |||
| Baseline | 21 (5.25) | 22.5 (6.5) | 0.878 |
| Day 90 | 25.5 (6.75) | 19 (2.5) | 0.033 |
| P-value d | 0.528 | 0.041 | - |
| Solid fats | |||
| Baseline | 21 (4.75) | 23 (7.5) | 0.697 |
| Day 90 | 22 (5.75) | 20 (7.5) | 0.477 |
| P-value d | 0.493 | 0.281 | - |
| Liquid oil | |||
| Baseline | 12.5 (4.25) | 12.5 (5.75) | 0.915 |
| Day 90 | 12 (5) | 11 (3) | 0.827 |
| P-value d | 0.636 | 0.311 | - |
| Tea and coffee c | |||
| Baseline | 4 (3) | 4 (3) | 0.971 |
| Day 90 | 4 (2.5) | 4.5 (1.75) | 0.662 |
| P-value d | 0.748 | 0.129 | - |
| Salty snacks c | |||
| Baseline | 16 (6) | 15 (6) | 1.00 |
| Day 90 | 14.5 (6) | 12 (3) | 0.534 |
| P-value d | 0.223 | 0.336 | - |
| Simple sugars c | |||
| Baseline | 16.5 (8.5) | 20 (8) | 0.182 |
| Day 90 | 23.5 (7) | 20 (6) | 0.145 |
| P-value d | 0.046 | 0.752 | - |
| Honey and jams c | |||
| Baseline | 7.5 (4) | 7.5 (3) | 0.739 |
| Day 90 | 7.5 (1.75) | 7 (3) | 0.456 |
| P-value d | 0.786 | 0.336 | - |
| Soft drinks c | |||
| Baseline | 5 (2.25) | 4 (2.25) | 0.436 |
| Day 90 | 4 (2.25) | 3 (1.75) | 0.345 |
| P-value d | 0.102 | 0.157 | - |
| Snacks and desserts | |||
| Baseline | 41.5 (9.75) | 45 (4) | 0.171 |
| Day 90 | 57 (14) | 48 (6) | 0.021 |
| P-value d | 0.007 | 0.096 | - |
a Values are expressed as median (IQR) for non-normal distribution.
b Between-group comparison was applied based on the independent sample t-test/Mann-Whitney U test.
c Non-parametric tests were applied to analyze data.
d Within-group comparison was applied based on the paired sample t-test/Wilcoxon Signed Ranks test.
5. Discussion
The findings of the present study showed that assa-foetida increased ESR values and decreased HDL-C serum levels at the end of the study compared to baseline. In addition, lower hs-CRP values were found in placebo-treated groups at the end of the study compared to baseline. Furthermore, assa-foetida increased the value of SGPT compared with the placebo at the end of the study.
5.1. Inflammatory Markers (ESR and High-sensitive C-Reactive Protein)
Assa-foetida increased ESR levels at the end of the study compared to baseline. Moreover, lower hs-CRP levels were found in the placebo group compared to baseline. These changes might reflect the influence of the patients’ dietary habits, particularly low nut and seed consumption and high refined grain intake in the assa-foetida group.
Several in-vivo studies have demonstrated the protective effect against tissue damage due to the antioxidant capacity, anti-hyperglycemic potential, and ability to regenerate β-cells of various species of Ferula in animal models of diabetes mellitus (4, 18-21). However, to our knowledge, this is the first clinical trial regarding the anti-diabetic potentials of F. assa-foetida. An increased ESR value in the assa-foetida-treated group at the end of the study compared to baseline and lower hs-CRP values in placebo-treated groups at the end of the study compared to baseline can be attributed to the negative effect of the common dietary pattern of the patients evaluated by FFQs on the observed outcomes related to T2DM. Believing that regular nut consumption and substituting nuts for refined grains may lead to a decrease in circulating levels of pro-inflammatory markers such as ESR (22) and CRP (23), it seems rational that less frequent consumption of nuts and seeds and more frequent consumption of refined grains in patients treated with assa-foetida on day 90 compared to baseline have negatively impacted the results of our study.
5.2. Lipid Profile (HDL-C)
A decrease in HDL-C levels was found in the assa-foetida group by the study’s end. The T2DM often comes with decreased levels of HDL-C, which adds to the heightened cardiovascular risk linked to this condition. Since HDLs can enhance glucose absorption by skeletal muscle and promote insulin secretion from pancreatic beta cells, it is possible that low HDL levels in type 2 diabetes might also worsen the management of the disease (24). Besides, dietary data showed a higher carbohydrate consumption (e.g., whole grains, refined grains, and potatoes) in the assa-foetida group, which might contribute to decreased HDL; as a low-carbohydrate diet is generally associated with improved HDL-C levels in T2DM (25). Moreover, diets rich in whole-grain foods tend to raise serum HDL-C levels. Whole-grain intake may also favorably modify antioxidant and inflammatory states. Whole-grain components that appear to make major contributions to these protective effects include dietary fiber, vitamins, minerals, antioxidants, phytosterols, and other phytochemicals (26).
The present study found no significant differences between assa-foetida and placebo-treated groups in the anthropometric and clinical characteristics of the patients at baseline and the end of the study period. An experimental study found evidence consistent with our finding, as prolonged oral administration of F. assa-foetida extracts at a dose of 250 mg/kg for 28 days did not show any significant alterations in body weight and body weight gain (27). The outcomes suggest that assa-foetida supplementation alone may not be sufficient to yield significant anti-inflammatory or antioxidant benefits. Positive dietary modifications, such as increased nut and seed consumption and reduced refined grain intake, could enhance the efficacy of the herb.
5.3. Liver Enzyme (Serum Glutamic Pyruvic Transaminase) and Safety Profile
The SGPT levels increased in the assa-foetida group compared to placebo, though they remained within the normal range (5 - 40 IU/L). This aligns with some experimental findings on mild hepatic changes associated with assa-foetida use (28, 29). Increased ALT was utilized as a proxy marker for Nonalcoholic Fatty Liver Disease (NAFLD), indicating that this condition might be prevalent among those with T2DM. Elements of metabolic syndrome, which frequently occurs prior to T2DM, such as obesity, hyperinsulinemia, insulin resistance, elevated triglyceride levels, and high blood pressure, have been suggested as potential contributors to NAFLD in previous research. This research aligns with the existing comprehension of NAFLD's development as a liver-related aspect of metabolic syndrome, as elevated ALT levels were prevalent among individuals diagnosed with T2DM, indicating that the emergence of NAFLD might occur before the onset of T2DM (30).
An experimental study observed little toxicity in the short-term use of F. assa-foetida extract and displayed mild changes by way of thrombosis, sinusoidal leukocytosis, and portal infiltration with inflammatory cells in the liver of treated rats, which showed a normal histological structure of the hepatic lobule, consistent with our study (27).
Regardless of the higher SGPT serum levels in the assa-foetida-treated group compared to the placebo at the end of the study, these levels were within the normal range (5 - 40 IU/L) before and after the study. Considering the common alteration in liver enzyme levels of T2DM patients (31), the increase in SGPT and ESR levels, though within normal ranges, raises concerns about potential hepatic and inflammatory effects. Lower doses may be more suitable for reducing these risks.
5.4. Glucose Metabolism, Comorbidities, and Dose Consideration
In this study, no significant differences in glucose metabolism or diabetes-related comorbidities were found between the assa-foetida and placebo groups. Previous studies have shown mixed results on the anti-hyperglycemic and anti-lipidemic effects of F. assa-foetida, often depending on the dosage, extraction methods, and duration of testing (20, 32, 33). It is well established that hyperglycemia and hyperlipidemia are common symptoms of T2DM, leading to modification of glucose and lipid metabolism and changes in liver enzyme levels (34).
Additionally, it has been established that ferulic acid is one of the phenolic phytochemical components found abundantly in assa-foetida (35), which has exhibited antidiabetic potential in animal models of diabetes by elevating insulin secretion (36), preventing intestinal α-glucosidase (37), and activating glucokinase (38). Even though hyperglycemia and hyperlipidemia are more common in supplementation with high doses (HD) than low doses (LD) of F. assa-foetida, the prescribed dose of 250 mg twice daily might have been too high. Evidence suggests that lower doses of ferulic acid may provide better glycemic and lipid control while minimizing adverse effects in diabetic models (20, 33, 39, 40).
5.5. Conclusions
Ferula assa-foetida supplementation at 250 mg twice daily increased ESR and SGPT levels (within normal ranges) and decreased HDL-C serum levels in T2DM patients. Combining assa-foetida with positive dietary changes may enhance its anti-inflammatory and antioxidant benefits. Lower doses (< 250 mg twice daily) could reduce potential hepatic and inflammatory complications. Future research with a larger sample size and more robust dietary interventions is recommended to confirm these findings. This study contributes to the growing body of research on the clinical potential of F. assa-foetida, while emphasizing the need for a holistic approach combining supplementation with dietary improvements for better management of T2DM.
5.6. Study Strength and Limitations
The strength of this study was the long duration of treatment to evaluate the long-term efficacy of F. assa-foetida in T2DM management. However, the study’s small sample size and the impact of the COVID-19 pandemic on enrollment limit the generalizability of the findings (41). A larger clinical trial is recommended to further evaluate the efficacy and safety of F. assa-foetida.