1. Background
Celiac disease (CD) is a multifactorial systemic disease that causes enteropathy after consuming gluten in genetically susceptible individuals. Its prevalence varies significantly across different ages, sexes, and geographic regions (1). Celiac disease arises from an increased immune sensitivity and reaction to a group of proteins known as gliadin, commonly found in the outer shell of grains such as wheat, rye, and oats. It is characterized by elevated circulating autoantibodies against tissue anti-transglutaminase (Anti-TTG Ab). Due to chronic inflammation of the small intestine from immune system attacks, CD is also referred to as immune enteropathy (2, 3).
Over the past decade, studies have shown a link between CD and a range of fertility-related disorders, indicating that CD can impact the reproductive health of women. Symptoms in women of reproductive age with CD may include delayed puberty and menstrual cycles, secondary amenorrhea, early menopause, and reproductive issues such as recurrent miscarriages, stillbirths, primary and secondary infertility, and intrauterine growth disorders (3-5).
Various mechanisms have been proposed to explain how celiac disease (CD) can lead to infertility. Active CD often results in chronic inflammation of the small intestine mucosa, causing histopathological changes such as crypt hyperplasia and villous atrophy. These changes lead to the malabsorption of micronutrients like zinc, iron, selenium, and folic acid, which are crucial for fertility. For instance, zinc deficiency can impair the production and secretion of luteinizing and follicle-stimulating hormones, leading to secondary amenorrhea, spontaneous miscarriage, and preeclampsia. Similarly, selenium deficiency may have comparable effects (6). A lack of folic acid can also adversely affect the nervous system (7). Additionally, delayed menarche and premature menopause have been identified as factors contributing to fertility issues in women with CD (8). Ludvigsson et al.’s study indicated that mothers with CD had lower placental weights than others (9). Furthermore, research suggests that Anti-TTG Ab generated in the endothelium promotes angiogenesis and causes apoptosis and delays in repair, thereby impairing maternal-fetal communication after implantation (10).
The literature indicates that women with CD who adhere to a long-term gluten-free diet experience the same length of fertile period as healthy women. Therefore, safe and inexpensive diagnostic and treatment options could be a viable solution for patients with undiagnosed CD who frequently visit infertility clinics and face high medical costs (11-13).
2. Objectives
Given the contradictory evidence and the lack of regular CD screening in infertility clinics, our study aimed to evaluate the frequency of CD in women visiting infertility clinics and to assess the association between increased Anti-TTG Ab levels and infertility.
3. Methods
3.1. Ethical Considerations
The current study was conducted in accordance with the Declaration of Helsinki and received approval and financial support from Shiraz University of Medical Sciences (grant No. 90.01.01.3399). It was also approved by the local Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1390.3399). To address ethical concerns, the collected data were kept confidential and only accessible to the researchers; therefore, patients' names were not disclosed.
3.2. Study Setting and Population
This case-control study was conducted on two groups of female patients of reproductive age (18 - 50 years old) referred to Ghadir Mother and Child Hospital in Shiraz, one of the primary obstetric centers in southern Iran (May-July 2021). The control group consisted of fertile women with at least one successful pregnancy and no previous history of miscarriage, repeated miscarriage, secondary infertility, or intrauterine growth restriction (IUGR), who visited the women’s clinic at the same hospital. The case group included infertile women who visited the infertility clinic during the study period for one of the following reasons: (1) unexplained infertility (infertility after one year of intercourse with common causes such as abnormal ovulation, anatomy, and uterine or tubal function, and semen abnormalities ruled out); (2) repeated miscarriage (three or more miscarriages before 12 weeks); or (3) failure of assisted reproductive technologies (three or more unsuccessful attempts at technologies such as in vitro fertilization (IVF)). Patients who did not consent to blood sampling or refused a duodenal biopsy despite a positive serology test were excluded from the study.
3.3. Study Protocol
After providing oral explanations about the study and obtaining written informed consent, anthropometric and clinical data were collected from all participants. A data collection form was completed, including demographic information, age at marriage, duration of infertility, presence of gastrointestinal symptoms, and history of CD-related autoimmune diseases in the individual or their family, such as Type I diabetes and autoimmune thyroid diseases. Subsequently, a 5-cc blood sample was taken to measure Anti-TTG Ab levels using a Monobind kit (USA) with an Enzyme-linked immunosorbent assay (ELISA). IgA levels were assessed using the Bonding site kit (UK) with the Nephelometry method. According to these kits, an Anti-TTG Ab level of < 12 was considered normal, 12 - 18 borderline, and ≥ 18 as positive. Patients with Anti-TTG Ab levels above the normal range, who did not have IgA deficiency, were referred to a gastroenterologist for a duodenal tissue sample.
3.4. Statistical Analysis
All analyses were conducted using SPSS version 26.0 for Windows. The Shapiro-Wilk t-test was utilized to assess the normal distribution of numerical variables. Depending on the normality, either independent sample t-tests or Mann-Whitney tests were applied for two-group comparisons of continuous variables. Fisher’s exact test was used for proportions. Results are presented as mean ± standard deviation (SD) for continuous variables and number (percentage) for categorical data. Logistic regression analysis was performed to assess the association between increased Anti-TTG Ab levels and infertility. All variables with a P-value < 0.1 in the univariate analysis, except for those with zero frequency, were included in the multivariable analysis. A two-sided P-value of less than 0.05 was considered statistically significant.
4. Results
A total of 300 patients were enrolled, with 100 assigned to the case group and 200 to the control group. The mean ± SD age was 35.50 ± 5.99 years in the case group and 27.69 ± 8.83 years in the control group, showing a statistically significant difference (P < 0.001). The Body Mass Index (BMI) also differed significantly between the groups (P = 0.008). As indicated in Table 1, neither group had a family history of CD. However, a family history of infertility in first-degree relatives was reported by 22 patients (22%) in the case group, but by none in the control group (P < 0.001). A family history of hypothyroidism was present in 23 patients (23%) of the case group, while it was absent in all individuals in the control group (P < 0.001). Significant differences were also observed between the two groups concerning the history of diabetes (P < 0.001), stillbirth (P = 0.036), and the type of infertility (P < 0.001). The median duration of primary and secondary infertility in the case group was 24 years (range 1 - 25 years) and 19 years (range 1 - 20 years), respectively.
| Variables | Case (n = 100) | Control (n = 200) | P-Value |
|---|---|---|---|
| Age (y) | 35.50 ± 5.99 | 27.69 ± 8.83 | < 0.001 |
| Height (m) | 160.89 ± 5.54 | 158.11 ± 5.22 | < 0.001 |
| Weight (m) | 70.06 ± 18.45 | 68.05 ± 6.73 | 0.569 |
| BMI (Kg/m2) | 27.01 ± 6.76 | 27.26 ± 2.78 | 0.008 |
| Positive family history | |||
| Celiac disease | 0 (0.00) | 0 (0.00) | - |
| Infertility | 22 (22.00) | 0 (0.00) | < 0.001 |
| IUGR | 2 (2.00) | 0 (0.00) | 0.110 |
| Hypothyroidism | 23 (23.00) | 0 (0.00) | < 0.001 |
| Diabetes type 1 | 17 (16.00) | 0 (0.00) | < 0.001 |
| Early menopause | 1 (1.00) | 0 (0.00) | 0.333 |
| Secondary amenorrhea | 2 (2.00) | 0 (0.00) | 0.110 |
| Still birth | 3 (3.00) | 0 (0.00) | 0.036 |
| Secondary infertility | 25 (25.00) | 0 (0.00) | < 0.001 |
| Type of infertility | |||
| Unexplained infertility | 50 (50.00) | 0 (0.00) | |
| Recurrent abortion | 22 (22.00) | 0 (0.00) | |
| RIF | 28 (28.00) | 0 (0.00) | < 0.001 |
As shown in Table 2, out of 100 patients in the case group, eight tested positive for serology (Anti-TTG Ab ≥ 18). Of these, five underwent endoscopy by a gastroenterologist, and four were confirmed to have CD via duodenal biopsy. Among the controls, one patient tested positive for serology but had a negative duodenal biopsy. Significant differences were observed between the two groups regarding CD confirmed by serology (P < 0.001) and biopsy (P = 0.012).
| Serology Test (%) | Case (n = 100) | Control (n = 200) | P-Value a |
|---|---|---|---|
| Positive (Anti-TTG Ab ≥ 18) | 8 (8.00) | 1 (0.50) | 0.001 |
| Duodenum Biopsy Recommended (%) | 8 (8.00) | 1 (0.50) | 0.001 |
| Duodenum positive Biopsy Result from Patients with Indication (%) | 4 (4.00) | 0 (0.00) | 0.012 |
The Results of Serology Test
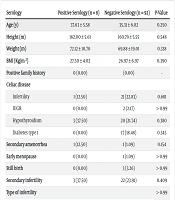
The characteristics and history of diseases were compared between subjects with positive and negative serology tests. No significant differences were found between the two groups in these factors (Table 3).
| Serology | Positive Serology (n = 8) | Negative Serology (n = 92) | P-Value |
|---|---|---|---|
| Age (y) | 37.63 ± 5.58 | 35.31 ± 6.02 | 0.250 |
| Height (m) | 162.00 ± 5.61 | 160.79 ± 5.55 | 0.548 |
| Weight (m) | 72.12 ± 10.70 | 69.88 ± 19.01 | 0.338 |
| BMI (Kg/m2) | 27.50 ± 4.02 | 26.97 ± 6.97 | 0.390 |
| Positive family history | 0 (0.00) | (0.00) | - |
| Celiac disease | |||
| Infertility | 1 (12.50) | 21 (22.83) | 0.681 |
| IUGR | 0 (0.00) | 2 (2.17) | > 0.99 |
| Hypothyroidism | 3 (37.50) | 20 (21.74) | 0.380 |
| Diabetes type 1 | 0 (0.00) | 17 (18.48) | 0.345 |
| Secondary amenorrhea | 1 (12.50) | 1 (1.09) | 0.154 |
| Early menopause | 0 (0.00) | 1 (1.09) | > 0.99 |
| Still birth | 0 (0.00) | 3 (3.26) | > 0.99 |
| Secondary infertility | 3 (37.50) | 22 (23.91) | 0.409 |
| Type of infertility | > 0.99 | ||
| Unexplained infertility | 4 (50.00) | 46 (50.00) | |
| Recurrent abortion | 2 (25.00) | 20 (21.70) | |
| RIF | 2 (25.00) | 26 (28.30) |
According to logistic regression analysis, there was a positive association between Anti-TTG Ab-confirmed CD and infertility (odds ratio (OR) = 17.30, 95% confidence interval (CI): 2.13 - 140.39, P = 0.008), even after adjusting for age and BMI (OR = 9.92, 95% CI: 1.17 - 84.21, P = 0.035). The multivariable regression model also showed a significant positive association between age and fertility (OR = 1.19, 95% CI: 1.08 - 1.16, P < 0.001) (Table 4).
| Independent Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95 % CI) | P-Value | OR (95 % CI) | P-Value | |
| Celiac disease (positive serology vs. negative) | 17.30 (2.13-140.39) | 0.008 | 9.92 (1.17-84.21) | 0.035 |
| Age (continues) | 1.19 (1.08 - 1.16) | < 0.001 | ||
| Body Mass Index (continues) | 1.00 (0.94 - 1.05) | 0.910 | ||
The Association Between Celiac Disease and Infertility
Due to conflicting evidence, neither gynecologists nor gastroenterologists currently recommend routine screening for celiac disease (CD) in infertile women (14, 15). Consequently, our study assessed the frequency of CD in this population and the association between CD and infertility to evaluate the necessity of screening.
Our findings indicated that 8% of infertile women tested positive for CD serology, with 4% confirming CD through both serology and duodenal biopsy. In contrast, only 0.5% of women in the control group tested positive for serology. These results suggest that women with Anti-TTG Ab-confirmed CD have an approximately tenfold increased risk of infertility, independent of age and BMI. However, due to the broad 95% CI, these findings should be interpreted with caution.
The reported frequency of CD among infertile women varies widely. Some studies suggest a higher prevalence in this group (3 - 8%) compared to the general population (16, 17), while others report rates similar to the general population (1.1 - 2.3%) (8, 11, 18). For instance, a 2011 study in Iran found an 8% prevalence of serology-positive CD among women with unexplained infertility, compared to 3.5% in the control group (18). A meta-analysis indicated that infertile individuals are three times more likely to have CD than controls (19). Conversely, a 2017 cohort study in Canada by Gunn et al. found that among 197 patients with unexplained infertility, only one tested positive for Anti-TTG Ab and confirmed CD through biopsy, leading the authors to conclude that routine CD screening for women with infertility is unwarranted (20). Similarly, a 2018 cohort study by Juneau et al. in New Jersey reported that the prevalence of CD among women undergoing IVF was similar to that in the general population (2.8%) (21). Furthermore, a recent meta-analysis found that only 0.7% of women with any form of infertility had biopsy-confirmed CD, a rate not significantly different from the general population (22).
There are several reasons for the discrepancies in the reported prevalence of celiac disease (CD) across studies:
- Selection of case groups: Some studies exclusively select women with unexplained infertility as the case group, while others include all infertile women, impacting the prevalence rates observed.
- Differences in diagnostic tests: Variations in diagnostic approaches can lead to different prevalence rates. Some studies rely solely on serology, whereas others confirm serology results with biopsies.
- Types of antibodies used: The choice of antibodies can affect detection rates. Some studies use IgA and Anti-TTG/antiendomysium antibodies, while others use IgA anti-gliadin or anti-PDG. Generally, the prevalence of positive serology is slightly higher with Anti-TTG compared to antiendomysium antibodies (19).
- Geographical variations: The relationship between the prevalence of CD and geographic region has been well-documented. This geographic variability can significantly influence prevalence rates.
- Variations in age groups: Since the prevalence of CD can vary by age, the selection of control groups that are appropriately age-matched is crucial for accurate comparisons (1).
- Influence of other conditions: Conditions like selective IgA deficiency and autoimmune diseases such as hypothyroidism, diabetes, and antiphospholipid syndrome can also impact the prevalence of CD, potentially leading to its underestimation (19, 23).
These factors contribute to the varying prevalence rates of CD reported in different studies, reflecting the complexity of accurately assessing the association between CD and infertility across diverse populations.
The American College of Gastroenterology (ACG) recommends screening for celiac disease (CD) in women who exhibit a combination of gastrointestinal bleeding and infertility-related symptoms (24). Moreover, given the potential for restoring fertility and alleviating symptoms related to the reproductive system through a gluten-free diet, some studies advocate for screening all women presenting with various midwifery symptoms such as infertility, repeated miscarriage, amenorrhea, premature menopause, and intrauterine growth restriction (IUGR) for CD (25). Consequently, further research is necessary to explore the association between CD and female infertility.
One limitation of this study was the small sample size, attributed to the high costs of diagnostic tests. It is recommended that future studies receive more funding to examine a larger sample of patients. Additionally, selection bias is a concern in case-control studies and may lead to inaccurate prevalence estimations. Patients with clinical symptoms or a family history of CD may be more inclined to undergo diagnostic serology tests than others (26). Convincing patients to undergo a duodenal biopsy (endoscopy) to confirm CD also presented challenges, as three patients were unwilling. It is important to note that patients with positive serology are more likely to develop CD than the general population, and their biopsy results may become positive in the future, thus highlighting the need for biopsy.
5. Discussion
The results of this study demonstrated that the frequency of celiac disease (CD) among infertile women was significantly higher compared to fertile women, indicating that CD is an independent risk factor for female infertility. Given that this disease can be effectively managed with a gluten-free diet, which may improve antibody titers, intestinal histopathology, and potentially fertility potential, screening for CD is recommended for women visiting infertility clinics. Our findings are particularly significant for cases with undiagnosed CD, whose fertility may have been compromised for many years without overt symptoms.