1. Background
Colorectal cancer (CRC) is a global health concern. It is the third most prevalent cancer and the second leading cause of cancer-related deaths worldwide (1, 2). In 2020, the estimated global mortality rate for CRC was over 900,000 (1). Research indicates a rising incidence of CRC internationally, particularly in developing countries (3). In 2020, the estimated age-standardized incidence rate of CRC in Iran was 12.0 - 19.1 per 100,000 in both sexes (2). Several studies have confirmed that CRC incidence in Iran has increased over recent decades. This trend is expected to continue, contributing to the rising burden of CRC due to the current high incidence and mortality rates (4, 5).
Colorectal cancer occurrence is associated with both genetic and acquired (environmental) factors. While genetic factors have a significant impact, modifying environmental factors can be highly effective in prevention (6). Modifiable risk factors for CRC include smoking, diet, obesity, low physical activity, night shifts, and alcohol consumption. Non-modifiable risk factors include age, male sex, personal or family history of CRC or polyps, race, high-risk genetic syndromes, and diseases such as diabetes and inflammatory bowel disease (IBD) (7, 8).
Common signs and symptoms of CRC include abdominal pain, rectal bleeding, an abdominal mass, changes in bowel habits (such as diarrhea or constipation), iron deficiency anemia (IDA), and unexplained weight loss. Among these manifestations, rectal bleeding is associated with the highest odds ratio for CRC (9, 10).
Screening can reduce the mortality rate and incidence of CRC by up to 60% when using methods such as colonoscopy and fecal occult blood testing (FOBT) (11). Therefore, CRC is considered one of the most preventable cancers and can be detected early through routine screening (12). The long development period from early stages, such as polyps, provides an opportunity to improve CRC prognosis (13). Since a significant portion of CRC etiology is related to modifiable factors, primary prevention is crucial. Given that most CRC cases develop slowly and originate from precursor lesions like adenomatous polyps, secondary prevention through screening methods—including stool-based tests, non-invasive imaging, and endoscopic procedures—is vital for healthcare providers (6). Many studies have identified the lack of advice from medical doctors and healthcare workers as a major barrier to CRC screening in Asian countries (14-17). It has been shown that Asian doctors provide less advice compared to their American counterparts (18). However, even in developed countries, CRC screening performance is often inadequate (19). Numerous studies have indicated that the absence of physicians' recommendations significantly impacts CRC screening rates in Iran (20-24). Most average-risk individuals in Iran are unaware of available CRC screening tests and do not receive screening advice or information about CRC risk factors from their physicians (25). In a study conducted in western Iran, the general population considered the lack of physician advice to be the most significant health system-related barrier to CRC screening (20). Additionally, previous research in Hamadan found that physician support, as a component of social support, was a significant predictor of CRC screening intention (P < 0.05) among average-risk patients (26). Some studies have examined the relationship between physicians’ knowledge of CRC screening and their characteristics, such as age, gender, and post-graduation education (16, 22). However, there is limited relevant data available in Iran. These findings underscore the critical need for further evaluation of Iranian physicians’ knowledge and attitudes toward CRC screening.
Given the critical impact of screening on CRC incidence, mortality, and prognosis, overcoming barriers to screening programs is essential. One of the most important factors for an effective screening program is physicians' knowledge.
2. Objectives
This study aimed to assess family physicians' understanding of CRC screening standards, signs and symptoms, and risk factors. Additionally, we investigated variables that might influence their knowledge, including age, gender, place of employment, work experience, and time since graduation. By addressing gaps in knowledge, we can implement effective interventions to improve it.
3. Methods
In this cross-sectional study, we evaluated family physicians' knowledge of CRC in Hamadan, Iran, from June to September 2017. The questionnaire was designed based on the latest updates from the National Comprehensive Cancer Network (NCCN) guidelines (27, 28). We modified the questionnaire to align with Iran's package of essential non-communicable diseases (IraPEN) (29), ensuring it conformed to the national CRC screening program of Iran. The content validity of the questionnaire was confirmed by consulting with a group of nine experts from various fields, including epidemiologists, oncologists, gastroenterologists, and community and preventive medicine specialists from the cancer registration unit at the Hamadan Province Health Center. The mean Content Validity Index (CVI) and content validity ratio (CVR) were reported as 0.9 and 0.95, respectively. We piloted the questionnaire with a random sample of 20 physicians, calculating the Cronbach alpha to assess its reliability, which yielded a score of 0.708, demonstrating that the questionnaire was reliable.
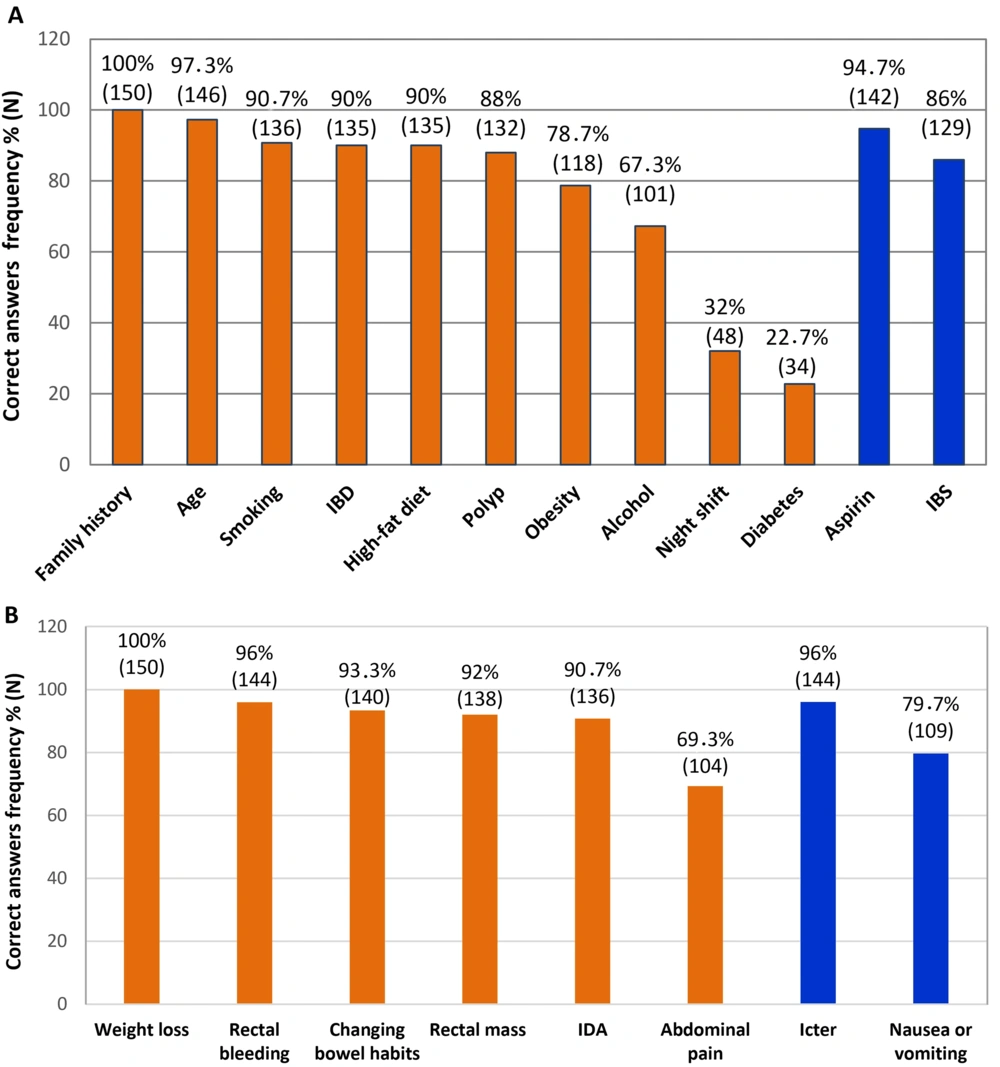
After obtaining approval, we used a convenience sampling method to distribute 200 questionnaires to physicians employed by the Hamadan province health center. We excluded physicians who did not complete the questionnaires (n = 28) or who had received education about CRC screening and risk factors after graduation (n = 22). The questionnaire consisted of two main parts. The first part included questions about age, gender, workplace, years of work experience, and years since graduation. The second part contained 38 questions: 18 about CRC screening (Table 1), 12 about risk factors (Figure 1A), and 8 about signs and symptoms (Figure 1B). One point was awarded for each correct answer.
| Questions | Correct Answer, No. (%) |
|---|---|
| Age to start and stop screening in normal population | 79 (52.7) |
| Best screening method for moderate risk patients | 76 (50.7) |
| Screening intervals for each method | 103 (68.7) |
| Screening onset age, intervals and methods for patients with a family history of CRC in first degree relatives < 60 years old | 108 (72.0) |
| Screening onset age, intervals and methods for patients with a family history of CRC in first degree relatives > 60 years old | 40 (26.7) |
| Screening onset age, intervals and methods for patients with a family history of CRA in first degree relatives < 60 years old | 63 (42.0) |
| Screening onset age, intervals and methods for patients with a family history of CRA in first degree relatives > 60 years old | 44 (29.3) |
| Screening onset age, intervals and methods for patients with a family history of CRC/CRA in second/third degree relatives | 92 (61.3) |
| Screening intervals for patients with personal history of hyperplastic, left-sided, sessile, and < 1 centimeter in diameter CRA | 21 (14.0) |
| Screening intervals for patients with personal history of hyperplastic, right-sided, sessile, and < 1 centimeter in diameter CRA | 34 (22.7) |
| Screening for patients with personal history of pedunculated or adenomatous CRA | 96 (64.0) |
| Screening onset age, intervals and methods for relatives of FAP patients | 64 (42.7) |
| Diagnostic criteria for HNPCC | 19 (12.7) |
| Screening onset age, intervals and methods for relatives of HNPCC patients | 50 (33.3) |
| Screening intervals for recently treated CRC patients who had not undergone colonoscopy before surgery | 82 (54.7) |
| Screening intervals for recently treated CRC patients who had undergone colonoscopy before surgery | 46 (30.7) |
| Screening intervals for patients who underwent successful treatment > 3 years ago | 85 (56.7) |
| Screening and surveillance in IBD patients | 66 (44.0) |
Abbreviations: CRC, colorectal cancer; CRA, colorectal adenoma; FAP, familial adenomatous polyposis; HNPCC, hereditary nonpolyposis colorectal cancer; IBD, inflammatory bowel disease.
Knowledge of family physicians towards colorectal cancer (CRC) risk factors and signs and symptoms. Frequency of correct answers are presented. A, notice that aspirin and irritable bowel syndrome (IBS) were considered as negative risk factors; also, B, icterus and nausea/vomiting were asked to make the participants think of other diagnoses rather than CRC.
We described continuous variables using frequencies and percentages, and quantitative variables with means and standard deviations. Statistical analysis was performed using SPSS 21. The Kolmogorov-Smirnov test was utilized to analyze the distribution of the data. Since the data did not follow a normal distribution, we employed nonparametric statistical tests, including Mann-Whitney U, Kruskal-Wallis, and Spearman’s correlation tests, to compare the scores of physicians' knowledge across different variables. Associations between knowledge scores and physicians’ gender or workplace location were investigated using Mann-Whitney U and Kruskal-Wallis tests, respectively. Additionally, Spearman’s correlation test was used to assess the relationships between physicians' knowledge and their age, years since graduation, and work experience. A multiple regression model was used to identify predictors of the total knowledge score. A P value of < 0.05 was considered statistically significant.
The ethics committee of Hamadan University of Medical Sciences approved this study [IR.UMSHA.REC.1396.393]. All physicians were thoroughly informed and provided ethical consent prior to participation. The STROBE checklist for cross-sectional studies was used when writing our report (30).
4. Results
Out of a total of 200 questionnaires, 150 were completed after three months, resulting in a 75% response rate. The sociodemographic characteristics of the 150 family physicians who answered the questionnaires are summarized in Table 2. About 47% of them were male and 53% were female. Approximately a quarter (24%) of them worked in the provincial capital, Hamadan city. Their mean age was 35.4 years, ranging from 25 to 55 years. The mean time since graduation and work experience were 9.43 and 8.67 years, respectively.
| Variables | Values |
|---|---|
| Age (y) | |
| Range: 25 - 55 | 35.4 ± 8.15 |
| Gap after graduation (y) | |
| Range: 1 - 26 | 9.43 ± 7.87 |
| Work experience (y) | |
| Range: 1 - 26 | 8.67 ± 7.45 |
| Gender | |
| Male | 70 (46.7) |
| Female | 80 (53.3) |
| Place of employment | |
| Capital of province | 36 (24.0) |
| Country | 114 (76.0) |
a Values are expressed as mean ± SD or No. (%).
Participants answered 38 questions regarding CRC signs and symptoms, screening, and risk factors. The total mean score was 25.11 ± 4.64. Although the mean scores for the signs and symptoms (7.1 ± 0.81 out of 8) and CRC risk factors (10.29 ± 1.83 out of 13) were acceptable, the physicians demonstrated insufficient knowledge about the CRC screening program. Their mean score in the screening section was less than half of the total possible score of 18 (7.77 ± 3.43). Table 3 provides these results in detail.
| Knowledge | No. (%) | Mean ± SD | Range |
|---|---|---|---|
| Screening program | 18 (43.16) | 7.77 ± 3.43 | 2 - 16 |
| Risk factors | 13 (79.15) | 10.29 ± 1.83 | 5 - 13 |
| Signs and symptoms | 8 (88.75) | 7.10 ± 0.81 | 5 - 8 |
| Total | 38 (64.38) | 25.11 ± 4.64 | 16 - 35 |
The results of the CRC screening questionnaire are listed in Table 1. Family doctors who participated in our study performed best on questions regarding the screening program for patients with a family history of CRC in first-degree relatives aged under 60 years (72%). In contrast, they had limited information about the diagnostic criteria for Lynch syndrome (12.7%).
Analysis of the risk factors section of the questionnaire revealed that only 22.7% and 32% of participants recognized diabetes and night shifts, respectively, as CRC risk factors. In contrast, the majority of participants had more information about other risk factors: Family history (100%), age (97.3%), smoking (90.7%), IBD (90%), high-fat diet (90%), polyps (88%), obesity (78.7%), and alcohol (67.3%) (Figure 1A). Additionally, most participants were aware of aspirin consumption (94.7%) and irritable bowel syndrome (86%) as factors that reduce risk (Figure 1A, blue columns).
Our results indicate that participants had strong knowledge of CRC signs and symptoms, including weight loss (100%), rectal bleeding (96%), changing bowel habits (93.3%), rectal mass (92%), and IDA (90.7%). However, nearly 70% identified abdominal pain as a symptom of CRC (Figure 1B). The questionnaire included questions about icterus and nausea/vomiting to prompt participants to consider other diagnoses beyond CRC (Figure 1B, blue columns).
Spearman’s correlation coefficient test was used to explore the relationship between demographic characteristics and family physicians' total scores. Statistically significant reverse correlations were observed between participants' knowledge and their age (r = -0.342; P < 0.001), years spent after graduation (r = -0.228; P = 0.005), and years of work experience (r = -0.247; P = 0.002). Multiple regression analysis revealed that only age was a significant independent predictor of a lower total knowledge score (P = 0.002). The Mann-Whitney U test was used to investigate the relationship between participants' knowledge scores and their gender, showing no statistically significant results (P = 0.929). Additionally, no significant relationship was found between knowledge and place of employment using the Kruskal-Wallis test (P = 0.399).
5. Discussion
Colorectal cancer is a significant health concern worldwide and ranks among the leading causes of cancer-related deaths. The prognosis and survival rate of CRC largely depend on various factors, including the stage of cancer at diagnosis. Early-stage diagnosis allows for more successful treatment and better outcomes, making regular screening crucial in reducing the burden of CRC (12, 20). Previous research has indicated that knowledge, attitude, and behavior towards CRC screening in Iran are moderate to poor (31). Majidi et al. published a review on cancer screening awareness and practices in Iran, highlighting poor knowledge about CRC risk factors and screening. They noted that only 5% and 15% of average-risk individuals had undergone colonoscopy and FOBT, respectively (21). A prior study involving 477 average-risk patients in Hamadan identified physicians’ and family support (social support) as significant predictors of CRC screening intention (P < 0.05) (26). Therefore, evaluating physicians' knowledge about CRC is beneficial.
The current study's results indicated that while family doctors had acceptable knowledge of CRC signs and symptoms (89%) and risk factors (79%), their knowledge of CRC screening programs was limited (43%). These findings align with a similar study by Sabet et al., which found that although 90% of physicians knew about CRC signs and symptoms, only 17.3% were aware of the appropriate screening age based on NCCN guidelines. Our results also confirmed a negative correlation between physicians' age and their knowledge about CRC (r = -0.342, P < 0.001), consistent with the aforementioned study (r = -0.12, P = 0.03). Additionally, like our study, Sabet et al. did not find a significant difference in physicians' knowledge based on sex (P = 0.3) (22).
A cross-sectional study of 197 physicians in public primary care health centers in Malaysia reported that the mean score for CRC screening knowledge was insufficient (48.7% ± 17.7%), and this was significantly associated with postgraduate education (P < 0.001). These results highlight retraining courses as a promising method to address the decline in knowledge that occurs after graduation (r = -0.228; P = 0.005). The frequencies of correct answers to questions about screening in patients with IBD, a family history of hereditary nonpolyposis colorectal cancer (HNPCC), and familial adenomatous polyposis (FAP) were 49%, 60%, and 60%, respectively. However, our results showed relatively lower scores in these areas (44%, 33%, and 43%, respectively). Multivariate analysis indicated that physicians who perceived CRC screening as a cost-effective intervention practiced it more frequently (P = 0.001; OR = 3.3; 95% CI: 1.7 - 6.6), compared to those with greater knowledge (P = 0.185). This suggests that a positive attitude among general physicians may be more influential than knowledge in promoting CRC screening (16).
In 2019, a study of 581 medical students in years 4 to 6 in Saudi Arabia found that a positive attitude towards CRC screening predicted higher knowledge of CRC risk factors and screening (OR = 2.74; 95% CI: 1.86 - 4.03). Higher medical education level was an even stronger predictor (OR = 3.23; 95% CI: 2.01 - 5.18) (15). These findings demonstrate the significant impact of clinical practice during medical education on the effectiveness of screening programs.
Many studies have found that healthcare workers' recommendations are significantly associated with increased CRC screening awareness (17, 21-23, 32). A study by Bidouei et al. revealed that 95.8% of patients from a referral center in Iran had never been screened for CRC. Additionally, more than 90% of them were unaware of CRC risk factors, signs, symptoms, and screening tests. Lack of physicians' recommendations was cited as the reason for 12.7% of them not undergoing any CRC screening tests (23). In 2022, Dolatkhah et al. conducted a study to identify the main barriers to participating in a population-based CRC screening program through an online questionnaire in East Azerbaijan, Iran. It was found that 35% of male and 47% of female participants considered the lack of recommendations from healthcare centers and physicians as the most common barrier among health system-related barriers. Additionally, 15% of males and 22% of females cited a lack of knowledge among staff and health workers as another barrier (20).
A cross-sectional study conducted by Al-Azri et al. in Oman showed that 76.3% of the adult population attending a teaching hospital had not heard about CRC screening. Although 93.9% of them had not undergone CRC screening, 52% reported that they would consider undergoing the screening in the future if advised by a physician (32).
In 2021, a study by Huang et al. assessed the knowledge, attitudes, and barriers related to CRC screening among high-risk populations in China. The study revealed that participants had poor knowledge about CRC. Although 70% of participants had a positive attitude towards CRC screening, only 13.3% had undergone the procedure. Additionally, the study found that individuals who had recently seen a doctor had more knowledge and a greater likelihood of undergoing screening tests. Lack of advice from physicians (cited by 29.8% of participants) and insufficient knowledge were identified as reasons for not undergoing screening (17).
A recent study highlighted that outcome expectancies, normative beliefs, and risk perception were strong predictors of CRC screening intention among the Iranian population. These findings underscore the importance of healthcare providers, especially physicians, in encouraging patients to undergo CRC screening by explaining the test process, preparation, and reassuring them about positive outcomes. Additionally, warning about the serious complications of a delayed or failed diagnosis can be an effective risk perception intervention (33).
Overall, family doctors play a critical role in CRC awareness by providing education, facilitating screening, assessing individual risk factors, empowering patients, coordinating care, and advocating for community-wide initiatives. Their involvement is instrumental in increasing awareness, early detection, and reducing the burden of CRC (17, 34). Patients who have received advice from a clinician are up to two times more likely to be aware of CRC screening (35). The influence of CRC screening educational courses on nurse practitioners showed an increase in their knowledge and a rise in screening rates by 4% (34). This can motivate health system policymakers to implement educational programs for general physicians.
Given the accessibility of family physicians within the Iranian healthcare system and their role in providing primary care services, they can play a central role in educating patients about CRC and preventive lifestyle changes. They are capable of performing risk assessments, conducting certain parts of the screening program, and referring patients who require further diagnostic or treatment measures (22).
Our study had some limitations. It was conducted at medical centers in Hamedan province, which are under the supervision of a single medical university that provided information to the study participants. Additionally, the exclusion of some physicians may have affected the generalizability of the results.
5.1. Conclusions
The results of the current study, similar to other relevant studies in Iran, indicated that despite acceptable knowledge about CRC signs and symptoms (88.7%) and risk factors (79.1%), family physicians generally had limited information about the CRC screening program (43%). Most participants did not consider diabetes and night shifts as risk factors for CRC. This study found a significant reverse correlation between physicians' knowledge of CRC risk factors, signs and symptoms, and screening with their age, years since graduation, and work experience, highlighting the need for retraining courses. Further studies are suggested to explore the precise relationship between physicians' knowledge and their recommendations about CRC screening. Additionally, the effects of proper education for physicians on CRC screening rates and general population awareness should be investigated.