1. Context
1.1. Rationale
Artificial intelligence (AI) refers to the development of sophisticated computer systems and programs that emulate functions typically requiring human cognitive abilities such as learning, reasoning, decision-making, and problem-solving (1). Over the past few decades, AI has gained significant traction in the medical field, especially in addressing major global health issues, such as diabetes mellitus and cancer (2, 3). Moreover, the demand for efficient healthcare delivery, while minimizing virus transmission during the COVID-19 pandemic, highlighted AI's potential in enabling digital medicine (4, 5). Artificial intelligence is expected to continue playing a pivotal role in the future of medicine.
Artificial intelligence in thyroidology has been explored from different perspectives, showing great potential, particularly in the diagnosis and management of thyroid nodules and cancers. Accurate detection and risk stratification of thyroid nodules can significantly aid clinicians in making informed decisions regarding further interventions (6). Thyroid nodules are becoming more prevalent in clinical practice, primarily due to the increased number of incidental diagnoses from the extensive use of advanced medical imaging techniques. While most thyroid nodules are benign, some exhibit malignant features (7). Over recent decades, thyroid malignancies have shown considerable growth among endocrine system cancers. Fortunately, most thyroid malignancies are less aggressive and can be managed effectively with timely surgical and medical interventions (8). However, delays in management can occur because thyroid cancers are often asymptomatic in their early stages, and there is a shortage of trained personnel and diagnostic resources, especially in underfunded healthcare settings (9).
This issue is exacerbated by the fact that conventional diagnostic methods are often time-consuming and prone to diagnostic errors. These errors can arise from the complexity of the diagnostic process or human cognitive biases and limitations. For example, the analysis of a fine-needle aspiration (FNA) biopsy may not always yield accurate or conclusive results due to inadequate sample preparation, the complexity of certain thyroid malignancies, and the subjective nature of the interpretative process, which heavily relies on the pathologist’s knowledge and expertise (10). Additionally, variations in thyroid test serum levels among different populations can add to this complexity (11). Incorporating AI into healthcare workflows could enhance diagnostic accuracy while reducing healthcare providers' workloads.
This study aims to review current AI applications in the diagnosis and management of thyroid nodules and thyroid cancers. We also explore the challenges and potential improvements needed for future AI implementation. The studies reviewed in this paper were selected based on their relevance, robust methodology, statistical significance, and broader coverage of related topics rather than focusing on a specific area in-depth.
1.2. Introduction to Artificial Intelligence Technologies
Artificial intelligence encompasses various subsets that drive innovation in medicine. Machine learning (ML), a fundamental subset of AI, focuses on developing systems that can recognize patterns in data without the need for pre-programming, unlike statistical models, and can make predictions or decisions based on new data. Machine learning is powerful in medicine by identifying logical relationships from large datasets sourced from electronic medical records, imaging results, clinical databases, and more (12). Deep learning (DL), a specialized branch of ML, employs an algorithmic architecture with multiple layers of artificial neurons, inspired by the structure and function of the human brain, to interpret vast amounts of unstructured data such as medical images, automatically learning their discriminative features (13). Natural language processing (NLP) allows computers to understand and respond to human language. Currently, NLP is gaining popularity for analyzing large amounts of unstructured medical textual data and using chatbot interfaces to answer queries from healthcare professionals and patients (14).
1.3. A Summary of the Approach to Thyroid Nodules
When a thyroid nodule is detected through palpation or as an incidental finding in head and neck imaging, a series of investigations are conducted to establish a definitive diagnosis and guide appropriate therapy. First, serum thyroid-stimulating hormone (TSH) levels are measured to assess the functional status of the nodules. Low serum TSH concentrations are indicative of hyperfunctional nodules, for which radioactive iodine (RAI) ablation therapy can be a relatively successful treatment option. Additionally, an ultrasound (US) examination is performed to evaluate the characteristics of the thyroid nodules and assess the risk of malignancy based on a standardized, evidence-based approach called thyroid imaging reporting and data systems (TI-RADS). The TI-RADS score determines whether a thyroid nodule requires a biopsy by identifying higher-risk features such as hypoechogenicity, microlobulation, irregular margins, microcalcification, and a taller-than-wide shape.
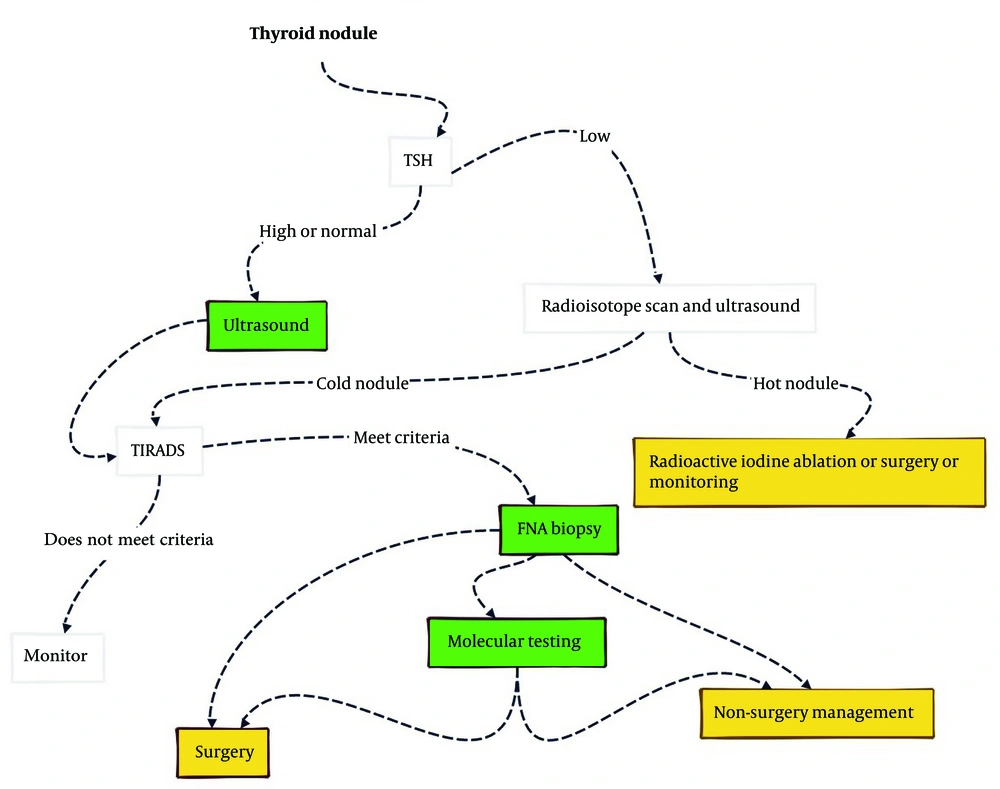
Subsequently, an FNA biopsy is performed, which is the gold standard for pre-operative definitive diagnosis and helps determine whether surgery or continued surveillance is necessary. The treatment depends largely on the FNA cytopathology and US findings. When the FNA biopsy result is indeterminate for malignancy, molecular testing can be utilized for diagnosis and to create individualized therapeutic plans (Figure 1) (15).
Diagnostic approach to thyroid nodule based on Harrison's principles of internal medicine. Green areas represent diagnosis topics, and yellow areas relate to treatment. This review focuses on the advancements of artificial intelligence (AI) in the highlighted topics (15).
2. Methods
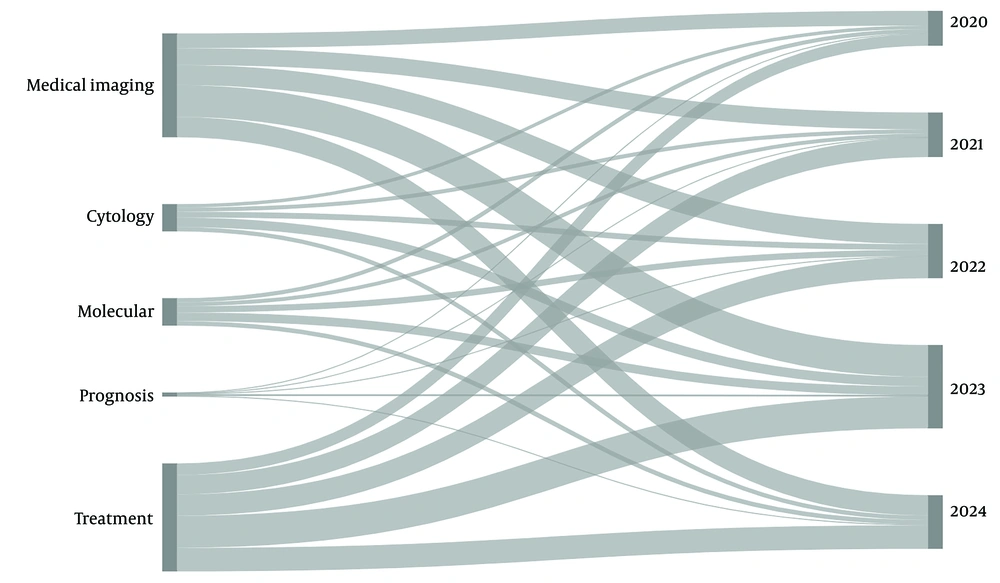
To review the most recent literature on AI applications in the assessment of thyroid nodules, we conducted a comprehensive search of articles published from January 2014 to March 2024 in the PubMed and Google Scholar databases. Our search was restricted to English, full-text articles focused on human subjects and classified as original studies, systematic reviews, and meta-analyses. We used various combinations of the following MESH terms: "Artificial intelligence", "machine learning", "deep learning", "thyroid nodule", "thyroid cancer", "diagnosis", "ultrasonography", "radiomics", "fine needle aspiration", "nuclear medicine", "molecular diagnostic techniques", "prognosis", "neoplasm staging", "therapeutics", and "thyroidectomy". Figure 2 illustrates the distribution of our search results across different aspects of AI applications in thyroid nodule evaluation over the past five years. The details of our search strategy are provided in Appendix in Supplementary File.
Titles and abstracts of the search results were screened to select relevant articles and gain a broad understanding of the topic and study designs. After full-text screening of the relevant articles, 45 studies were selected based on their robust methodology, statistical significance, and broader topic coverage, rather than an in-depth exploration of a specific area. Additionally, two authors manually reviewed the references to ensure inclusion of any further pertinent sources.
3. Results
We divided our investigations into three main sections to cover all aspects of AI applications in thyroid nodules: Diagnosis, prognosis, and treatment. We also summarized the selected studies used for writing this review in Table 1.
| Year | Aim | Technique | Dataset | Sample Size | Performance Metrics (Testing Cohort) | Reference |
|---|---|---|---|---|---|---|
| 2024 | Using AI for US multi-tissue segmentation | DL (UNet) + attention gating and pyramid pooling modules | US images | 4600 US images with ≥ 23000 annotated regions | DSC 81.88 | (16) |
| 2021 | Using AI for dynamic CEUS TNOD diagnosis | DL (hierarchical temporal attention network) | CEUS images from 1 center | 336 lesions’ CEUS images (77 Nodular Goiter, 84 Adenoma, 101 PTC, and 74 PTMC) | ACC 80.18 and F1 score 79.90 | (17) |
| 2023 | Using AI for US TNOD segmentation | DL (UNet) + boundary-preserving assembly transformer | A US images public dataset (TN3k) and a private one from 1 center | Public 3493 and private 328 US images | TN3k: ACC 97.22, F1 score 84.23, DSC 83.64, and AUC 92.03/private: ACC 97.80, F1 score 85.81, DSC 85.63, and AUC 92.19 | (18) |
| 2023 | Using AI for US TNOD segmentation | DL (BTNet: Boundary attention transformer net) | A US images public dataset (DDTI) and a private one from 6 centers | DDTI 626 and private 532 US images | DDTI: DSC 0.757/private: DSC 0.892 | (19) |
| 2019 | Comparing Linear and nonlinear ML models for TNOD classification | Ridge-penalty, Lasso-penalty, Elastic net, RF, k-SVM, ANN, k-NN, and NB | Pathological diagnosis confirmed TNODs US features: Size, margins, shape, aspect ratio, capsule, hypoechoic halo, vascularity, echo, cervical LN status calcification, and composition | 501 benign and 678 malignant | Overall AUC 0.928-0.954 (RF: AUC 0.989 training/0.954 testing) | (20) |
| 2019 | Using an ML model for US and USE malignant TNOD identification | LR, LDA, RF, k‐SVM, AdaBoost, k‐NN, ANN, NB, and CNN | US and USE features of 2064 TNOD underwent hemi‐ or total thyroidectomy from 1 center: USE grade, etc. | 1314 benign and 750 malignant | AUC 0.859-0.924 (RF: AUC 0.986 training/0.924 validation) | (21) |
| 2020 | Using ML for US follicular adenoma and carcinoma differentiation | ANN and SVM | 10 features out of 96 radiomics features extracted from 348 US from 2 centers | 252 adenoma and 96 carcinoma | ANN: Sensitivity 32.3, specificity 74.1, and ACC 79.4/SVM: Sensitivity 90.1, specificity 41.7, and ACC 69.0 | (22) |
| 2022 | Using AI for US TNOD diagnosis based on ACR TI-RADS | CNN (InceptionResNetV2) | 10 features covering TI-RADS categorization extracted from 1588 US TNODs who underwent hemi‐ or total thyroidectomy from 2 centers | PTC 484, FTC 14, MTC 1, nodular hyperplasia 987, follicular adenoma 70, and thyroiditis 32 | AUC 0.91 | (23) |
| 2021 | Using DL for US benign and malignant TNOD differentiation | DL (ThyNet) | Training: 18049 US images from 2 centers/Testing: 4305 images from 7 centers | 22354 US images | AUROC 0.922 | (24) |
| 2022 | Using AI for US benign and malignant TNOD differentiation | Meta-analysis | PubMed, Cochrane Library, Embase, Web of Science, China Biology Medicine, and China National Knowledge Infrastructure | 25 studies with 17429 US TNOD images | Sensitivity 0.88 (0.85 - 0.90), Specificity 0.81 (0.74 -0.86), and AUC 0.92 (0.89 - 0.94) | (25) |
| 2020 | Comparing a CAD system performance and 3 levels of experienced radiologists in TNOD US evaluation | DL (S-Detect) | Patients evaluated with US and cytology for TNOD from 1 center | 197 patients | S-Detect: ACC 88.48, sensitivity 92, specificity 87.9, NPV 98.40 / 1-month and 4-year experienced radiologists: ACC 83.03, Sensitivity 64 and 72, Specificity 86.4 and 85, NPV 93.08 and 94.44/9-year radiologists: ACC 95.76, Sensitivity 84, Specificity 97.9, NPV 97.16 | (26) |
| 2020 | Comparing a CAD system performance and 4 levels (1, 4, 9, and 20 years) of experienced radiologists in TNOD US evaluation | DL (S-Detect) | Patients evaluated with US and cytology for TNOD from 1 center | 204 TNODs in 181 patients | S-Detect: ACC 77.0, sensitivity 91.3, specificity 65.2, NPV 90.1, AUC 0.782 / 1-year experienced radiologist: ACC 63.7 (with S-Detect: 75.0), sensitivity 95.7 (94.6), specificity 37.5 (58.9), NPV 91.3 (93.0), AUC 0.666 (0.767) / 20-year: ACC 84.8 (85.3), sensitivity 96.7 (97.8), specificity 75.0 (75.0), NPV 96.6 (97.7), AUC 0.859 (0.864) | (27) |
| 2020 | Using DL for CT cervical LNM diagnosis | CNNs: VGG16, VGG19, InceptionV3, InceptionResNetV2, DenseNet121, ResNet, DenseNet169, and Xception | 3838 axial CT images from 698 thyroid cancer patients (PTC 689, FTC 5, MTC 3, and PDTC 1) | 3606 benign and 232 malignant LNs | AUROC 0.846 (0.784-0.884) (Xception: AUROC 0.884 external/0.942 internal validations) | (28) |
| 2024 | Using ML for US radiomics LNM diagnosis | Meta-analysis | PubMed, Embase, Cochrane, and Web of Science | 27 studies (16410 thyroid cancer patients, 6356 with LNM) | SROC for clinical features 0.76, for radiomics features 0.84, and for both 0.81 | (29) |
| 2024 | Using AI for nuclear medicine radiomics in thyroid diseases assessment | Meta-analysis | PubMed, Scopus, and Web of Science | 17 studies with 9627 patients | Not reported | (30) |
| 2023 | Using DL for in situ adequacy screening of unstained FNAB samples | DL (FNA-Net): MTL classifier + Faster R-CNN, Inception-ResNet v2, U-Net, and TensorFlow Object Detection API | FNABs | 6 patients with 21 slides and 287 cytopathologic images | AUC 0.84 and F1 score 0.81 | (31) |
| 2022 | Using ML for ROI identification on FNA WSI | TL: CNN (VGG11) + supervised learning + ML classifier + Ordinal regression | Thyroidectomy specimens with a previous FNAB | 908 FNABs: 799 training/109 testing (84 benign and 25 malignant) | AUC 0.931 for WSI-TBS and 0.896 for ROI-TBS | (32) |
| 2016 | Using ML for TNOD malignancy risk evaluation | ANN + resilient back propagation training algorithm | FNABs and surgical specimens of patients who underwent thyroid resection | 345 patients | ACC 64.5 and AUC 0.72 | (33) |
| 2020 | Using ML for TNOD FNA evaluation | RBFN + image analysis algorithms | Thyroidectomy specimens with a previous FNAB | 41324 nuclear measurement by image analysis from 288 benign and 159 malignant patients | Sensitivity 81.4, specificity 90.0, and ACC 86.9 | (34) |
| 2018 | Using ML for FNA follicular adenoma and follicular carcinoma differentiation | ANN | FNABs | 48 FNABs | AUC 1 and ACC 100 | (35) |
| 2020 | Using ML for FNA malignancy prediction | TL: CNN (VGG11) + multiple instance learning + ML classifier + ordinal regression | Thyroidectomy specimens with a previous FNAB | 908 WSIs from 659 patients: 799 training/109 testing | AUC 0.932, Sensitivity 92.0, and Specificity 90.5 | (36) |
| 2022 | Using AI for image analysis of ThinPrep-prepared FNABs | GBM, ETC | 20 FNABs of AUS/FLUS cases and 20 FNABs of benign TNODs | 400 low-power (100x) and 400 high-power (400x) images | AUC 0.75 for low- and 0.74 for high-power | (37) |
| 2023 | Using DL for FNA diagnosis | CNN (EfficientNetV2) + data augmentation + Gradient-weighted Class Activation Mapping (Grad-CAM) + stochastic neighbor embedding (t-SNE) | 393 FNABs | 148395 microscopic images of FNAB | AUC 0.49 for PDTC and 0.91 for MTC, others AUC > 0.95 | (38) |
| 2022 | Using AI for protein-based TNOD classification | ANN + feature selection and feature importance evaluation algorithms | 19 protein biomarkers from 1724 thyroid tissue samples proteomes | 1161 TNODs from 1133 patients compromising 288 TNODs for retrospective and 294 for prospective external validations | ACC 91 for training, 89 for retrospective, and 85 for prospective validations | (39) |
| 2021 | Using ML for predictive and diagnostic power of PPARγ targets for PTC evaluation | PPARGi: ML-Powered Personalized Scoring Index comprising 10 PPARγ targets, RF, SVM, k-NN, ANN, and LR | Datasets selected from public functional genomics data repository: TCGA-THCA, MMD-THCA (PTC), and MMD-THCA (ATC) | Three pairs of monozygotic twins with PTC | AUC 0.828 - 0.998 | (40) |
| 2022 | Using DL for drug response prediction by integrating bulk and scRNA-seq data | DL, TL | 6 public scRNA-seq datasets: GDSC, CCLE, etc. | 1280 cancer cell lines, 1557 drugs/chemical compounds, and their expression profiles on 15962 genes | F1 score 0.892 and AUROC 0.898 | (41) |
| 2023 | Using AI for CT radiomics ATC/PDTC from DTC differentiation | RF | Thyroid cancer patients underwent CECT from 1 center | ATC/PDTC 32 and PTC 58, FTC 40 | AUROC radiomics features 0.883, radiomics and clinical 0.908, ACC 84.6% and 86.5% | (42) |
| 2020 | Using ML multiparametric MRI radiomics for PTC aggressiveness prediction | 22 ML algorithms including LR, SVM, GBC, etc. | 120 TNOD patients underwent MRI and hemi- or total thyroidectomy from 1 center | 1393 MRI features from 71 non-aggressive and 49 aggressive | LASSO feature selection + GBC: AUC 0.874 training and 0.915 testing | (43) |
| 2021 | Using ML for PTC central LNM prediction based on preoperative and intraoperative clinicopathological characteristics | LR, GBM, XGBoost, RF, DT, and ANN | T1-T2, cN0 PTC patients underwent thyroidectomy from 1 center | 619 central LNM- and 652 central LNM+ | AUROC 0.695 - 0.750 (XGBoost 0.750) | (44) |
| 2020 | Using ML for PTC central LNM prediction based on clinical characteristics and US features | RF, ANN, DT, GBDT, XGBoost, and AdaBoost | 22 variables from 1103 patients who underwent thyroidectomy from 1 center | 491 central LNM- and 612 central LNM+ | AUC 0.680-0.731 (GBDT: AUC 0.731) | (45) |
| 2022 | Using ML for lung metastasis prediction based on clinicopathological characteristics | SVM, LR, XGBoost, DT, RF, and k-NN | Demographical and clinicopathological data from 9950 thyroid cancer patients from the SEER database: TN stage, age, sex, race, laterality, year of diagnosis, histological type, and LM | 212 lungM+ and 9738 lungM- | RF: ACC 0.99, F1-score 0.72, and AUC 0.99 | (46) |
| 2021 | Using ML for bone metastasis prediction based on clinicopathological characteristics | LR, RF, AdaBoost, DT, NB, SVM | Demographical and clinicopathological characteristics from 17138 thyroid cancer patients from the SEER database: Marital status, insurance status, grade, etc. | 166 boneM+ and 16972 boneM- | RF: AUC 0.917 and ACC 0.904 | (47) |
| 2021 | Using ML for recurrence prediction based on EMRs | Inductive logic programming | Information extracted from EMRs of 783 patients with >= 5 years F/U after total thyroidectomy, central LN dissection, and RIAT from 1 center | 54 recurrences and 729 recurrence-free | ACC 71.4 | (48) |
| 2022 | Using ML for FTC prognosis prediction | XGBoost, LightGBM, RF, LR, AdaBoost, GaussianNB, KNN, SVM, and MLP | 11 variables from the SEER database: Region, surgical methods, lymphadenectomy, TNM stage, etc. | 6891 patients with FTC with a median F/U of 64 months | XGBoost: AUROC 0.886 | (49) |
| 2023 | Using AI and bioinformatics for LNM prediction by key genetic variations and endocrine-disrupting chemicals | Different R packages with LASSO, SVM, and RF | Immune cell abundance identifier (ImmuCellAI), genomics of drug sensitivity in cancer (GDSC), and Human Protein Atlas (HPA) databases | 12 hub genes: ERBB3, etc. | ERBB3 as a diagnostic marker for thyroid cancer (AUC = 0.89), high LNM potential (AUC = 0.75), and LNM+ (AUC = 0.86) | (50) |
| 2023 | Using ML for PTC prognosis prediction based on molecular identifiers | ML (HighLifeR) | 502 cases annotated by the Cancer Genome Atlas Project | 82 genes: BRAFV600E, RAS, EZH2-HOTAIR pathway mutations, etc. | Not reported | (51) |
| 2022 | Using AI for guided US therapeutic effect evaluation of thoracoscopic thyroidectomy on PTC | MVA, DAS | Patients diagnosed with PTC by imaging or FNAB | Experimental: 94 patients, control: 119 patients | P < 0.05, not reported | (52) |
| 2021 | Using DL for recurrent laryngeal nerve identification during thyroidectomy | CNN (ResNeXt50-32 × 4d) + Mask R-CNN | Various images of recurrent laryngeal nerve and surrounding tissues | 277 images of 130 patients | DSC 0.707 | (53) |
| 2024 | Using DL for parathyroid gland identification during endoscopic thyroidectomy | DL: YOLOX | 838 endoscopic thyroidectomy videos | 32482 images extracted from videos | P < 0.001, not reported | (54) |
| 2023 | Using AI for the severity of postoperative scars prediction | CNN (ResNet-50) + convolutional block attention module + t-SNE + Grad-CAM | Images of thyroidectomy scars and clinical data | 1283 patients | ROC-AUC 0.896 for imaging and 0.912 for imaging and clinical features | (55) |
| 2023 | Using DL for RAIT dosimetry optimization | ANN + adam optimizer | DTC underwent RAIT dosimetry, using images and blood sampling gathered from the initial 4, 24, and 48 hr post administration | 83 patients | P = 0.351, not reported | (56) |
| 2024 | Using ML for RAIT success prediction in low-risk PTC by clinical data and radiomics | LR with lasso and ridge | Characteristics of low-risk PTC patients who underwent total or near total thyroidectomy and RAIT: Age, sex, and pre-ablative serum Tg | 130 patients | AUC 0.78 | (57) |
| 2018 | Using ML for thyroid cancer patients' family members' radiation exposure dose estimation | ANN | Characteristics of RAI-treated TC patients: Age, gender, home area, education, BMI, release dose rate, administrated residual activity, etc. | 99 family members of 52 patients | ROC-AUC 0.957 | (58) |
| 2022 | Using AI for targeting AKT1 peptide design for ATC treatment | Not reported | Peptide synthesis datasets | 96 plates | IC50 18.2 mM in 8303C and 12.4 mM in 8505C cells | (59) |
| 2023 | Using AI for new therapeutic target identification (Kir5.1) | Deep docking (VirtualFlow) | Gene Expression Omnibus, Cancer Genome Atlas, and TCGA databases, etc. | 68 pairs of primary tumors and para-tumor tissues (6 benign, 36 PTC, and 26 PTMC) | Not reported | (60) |
Abbreviations: RF, random forest; SVM, support vector machine; ANN, artificial neural network; k-NN, k-nearest neighbor; NB, naive bayes; LR, logistic regression; LDA, linear discriminant analysis; CNN, convolutional neural network; GBM, gradient boosting machine; DT, decision tree; GBDT, gradient boosting decision tree; MLP, multilayer perceptron; MTL, multi-task learning; TL, transfer learning; RBFN, radial basis function network; ETC, extra tree classifier; MVA, minimum variance algorithm; DAS, delay-and-sum algorithm; TNOD, thyroid nodule; LNM, lymph node metastasis; PTMC, papillary thyroid microcarcinoma; PDTC, poorly differentiated thyroid carcinoma; DTC, differentiated thyroid carcinoma; TC, thyroid cancer; anaplastic thyroid cancer (ATC), anaplastic thyroid carcinoma; TBS, thyroid cytopathology Bethesda system; AUS, atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; FNAB, fine needle-aspiration biopsy; DSC, dice similarity coefficient; ACC, accuracy; AUC, area under the curve; SROC, summary receiver operating characteristics ; ROC-AUC, area under the receiver operating characteristic curve.
3.1. Diagnosis
3.1.1. Artificial Intelligence Application in Medical Imaging
Like innovative US methods such as 3-dimensional US and contrast-enhanced ultrasonography (CEUS), systems based on a combination of AI with both conventional and novel US methods have shown considerable promise in augmenting medical imaging tasks (16, 17).
Image segmentation is an imaging preprocessing task that facilitates the localization of the region of interest (ROI) and delineation of its perimeters. Numerous studies have highlighted the superiority of AI-driven techniques for this purpose by incorporating detailed, multi-scale contextual features extracted from two-dimensional US images—features previously not visible to the radiologist's naked eye. Most of these studies have used convolutional neural networks (CNN), such as U-Net as the backbone, a visual transformer, and various modules like attention or feature fusion modules to improve the accuracy of thyroid nodule segmentation tasks. The performance of these models is typically assessed across public datasets and reported using the Dice similarity coefficient metrics (16, 18, 19).
Several studies have researched AI performance in detecting and classifying thyroid nodules in US images. Some ML algorithms, like random forest classifiers and artificial neural networks, have shown comparable performance to radiologists, with an area under the receiver operating characteristic (AUROC) curve ranging from 0.60 to 0.95 (20-22). Additionally, various CNN-based models have been developed to classify thyroid nodules, demonstrating diagnostic outcomes similar to those of clinicians (17, 23, 24). A meta-analysis validated the classification effectiveness of ML, DL, and computer-aided diagnosis (CAD) systems-assisted thyroid ultrasonography, reporting high performance indices: 0.92 (0.89 - 0.94) for the summarized receiver operating characteristic (SROC) curve, 0.88 (0.85 - 0.90) for pooled sensitivity, and 0.81 (0.74 - 0.86) for pooled specificity (25).
Due to this promising performance, commercially available, FDA-approved DL-based CAD systems, such as S-Detect (Samsung RS80A US system, Seoul, Korea), have emerged to assist in thyroid ultrasonography. S-Detect has demonstrated encouraging sensitivity compared to radiologists, although some studies have highlighted limitations in specificity and accuracy (61). For example, Chung et al. showed that S-Detect had superior sensitivity and negative predictive value (NPV) compared to junior radiologists, indicating that the system can effectively identify actual positive cases and provide reassurance when a negative result is predicted. This can reduce unnecessary FNA biopsies and false-negative cases for less experienced radiologists (26). However, the utility of such systems may be less pronounced for experienced radiologists with established diagnostic strategies (27). In settings without specialized professionals, S-Detect may be beneficial, but there is a risk of over-reliance on the system output, particularly by less experienced radiologists or healthcare professionals without senior oversight. These CAD systems are designed to work under radiologists' supervision, with manual input and settings adjustments required to optimize performance, and their output still requires interpretation by specialists (62).
With the emergence of NLP chatbots showing promising results in various healthcare fields (63-66), NLP-powered tools are now being used to generate radiology reports. Commercially available clinical decision support programs like RecoMD streamline recommendations based on radiological reports and patients' data (61).
Artificial intelligence has also been utilized to explore the relationship between ultrasonic characteristics and lymph node (LN) status. The integration of ML and DL with features derived from US and other modalities, such as ultrasound elastography (USE) and computed tomography (CT), has shown promise in classifying thyroid nodules and evaluating LNs (28, 29, 67). Radiomics, a powerful tool for extracting high-throughput quantitative features from medical images, has garnered significant attention in thyroid nodule risk stratification (68). Zhang et al.'s meta-analysis indicated that US-based radiomics AI models had a sensitivity, specificity, and SROC of 0.82, 0.84, and 0.76, respectively, for predicting LN metastasis in thyroid cancer patients (29). The potential of AI to create novel thyroid nodule risk stratification systems using radiomics and parameters beyond conventional US characteristics will be further explored in the prognosis section—AI application in staging system design.
The use of radiomics and AI in nuclear medicine is also expanding, with the potential to assess thyroid incidentalomas and cytopathologically inconclusive thyroid nodules by evaluating their secretory status and malignancy possibility. Studies have shown that ML and DL algorithms applied to various nuclear medicine imaging techniques can effectively differentiate benign and malignant nodules (30). However, methodological variations, differences in extracted features, limited training datasets, and a lack of external validations highlight the need for further research to facilitate the clinical implementation of AI in nuclear medicine.
3.1.2. Artificial Intelligence Application in Cytopathologic Diagnosis
An FNA biopsy is the most accurate method for diagnosing thyroid nodules, with the primary goal of distinguishing benign from malignant nodules and avoiding unnecessary thyroidectomy, given that only about 25% of thyroid cancer diagnoses are truly malignant (69). However, the accuracy of FNA diagnosis depends on sample quality, the pathologist's expertise, and standardized diagnostic criteria. These challenges can be addressed by implementing AI technologies to improve the precision and efficiency of thyroid malignancy discrimination (10).
One of the main challenges in FNA diagnosis is that approximately 10% of samples are non-diagnostic due to inadequate or poor-quality specimens, necessitating repeat biopsies. Jang et al. developed a hybrid DL model called FNA-Net to count follicular clusters in unstained samples and classify samples as non-diagnostic if the count falls below a specific threshold. This model achieved an AUC of 0.84 (31). Another study evaluated an ML algorithm designed to reduce pathologists' workload by detecting regions of interest (ROI) in whole slide images distorted by blood or dead space (32).
Several studies have shown AI-driven tools to be highly accurate in differentiating between benign and malignant nodules, with accuracy rates ranging from 64% to 100% (33-35). For instance, an ML algorithm trained on whole slide images achieved a sensitivity of 92%, specificity of 90.5%, and an AUC of 0.932, comparable to an expert pathologist (AUC: 0.931) (36). Another group developed a digital image analysis method using ImageJ software and Python's sklearn library to annotate indeterminate thyroid FNA biopsies (Bethesda III). This model, which outperformed pathologists (AUC of 0.75 vs. 0.62), demonstrated that AI and ThinPrep material could improve classification performance (37). However, some AI tools still face limitations in classifying poorly differentiated thyroid carcinoma (PDTC) from differentiated thyroid carcinoma (DTC) and in misclassifying medullary thyroid carcinoma (MTC) as benign, challenges even for professional pathologists (38).
3.1.3. Artificial Intelligence Application in Molecular Markers Testing
Although FNA biopsy is the most accurate pre-operative method for classifying thyroid nodules, around 30% of FNA biopsies are indeterminate, necessitating diagnostic thyroidectomy for final diagnosis. Molecular markers can help distinguish between benign and malignant nodules in these cases (70). AI has contributed to identifying molecular markers by analyzing large omics datasets to predict thyroid nodule malignancy risk. For instance, a neural network trained on proteomic analysis of 19 proteins extracted from tissue samples demonstrated accuracies of 85%, 89%, and 91% in retrospective and prospective multicenter cohort validations (39).
Artificial intelligence has also been applied to single-cell RNA-sequencing (scRNA-seq) technology to perform molecular-based classification of normal and cancerous cells. A model called Ikarus, developed using scRNA-seq technology, demonstrated high sensitivity and specificity when tested on several single-cell datasets of different cancers (71). Another study used scRNA-seq analysis of PPARGi coding genes by an ML algorithm, revealing that these genes are upregulated in papillary thyroid carcinoma (PTC) cells and can be used to identify this cancer type (40). Artificial intelligence applications in scRNA-seq are also valuable for predicting clinical prognosis and drug sensitivity, especially for PTC patients (72). Chen et al. developed an ML-based predictive algorithm for cancer drug response based on scDNA-seq data, identifying signature genes related to drug resistance mechanisms and assisting in targeted drug discovery (41).
3.2. Prognosis
3.2.1. Artificial Intelligence Application in Staging Systems Design
The management of potentially malignant thyroid nodules should be guided by various factors, including tumor type and size (T), lymph node involvement (N), distant metastasis (M), US characteristics, FNA biopsy results, and clinical and demographic data. AI and radiomics can enhance thyroid tumor staging in several ways. First, some studies have developed AI-powered systems to distinguish different types of thyroid tumors. For example, a random forest classifier demonstrated AUROC values of 0.88 and 0.90 for differentiating between poorly and well-differentiated thyroid carcinoma using radiomics features from contrast-enhanced CT alone and in combination with clinicopathological characteristics (42).
Second, substantial research focuses on developing models to detect invasive thyroid malignancies. Most of these models perform well in predicting extrathyroidal extension (ETE) and thyroid capsule invasion (TCI) in PTC by combining medical imaging radiomics features with clinical factors (68). For instance, a ML algorithm utilizing multiparametric magnetic resonance imaging (MRI) radiomics and clinical characteristics predicted PTC aggressiveness with an AUC of 0.92, whereas relying solely on clinical factors resulted in poor predictive performance (AUC: 0.56) (43).
Additionally, several studies have assessed ML-based tools for identifying key risk factors from demographic, clinical, biochemical, pathological, and imaging data that significantly contribute to central or lateral LN metastasis. Factors such as age, tumor size, multifocality, ETE, and suspicious US features have been identified as impactful in designing validated predictive models and scoring systems (44, 45). Numerous investigations have also focused on the predictive accuracy of ML models for forecasting distant metastasis in thyroid cancers, particularly bone or lung metastasis. These models, trained on large datasets containing demographic and clinicopathological information from cancer registries like surveillance, epidemiology, and end results (SEER) and the National Institutes of Health (NIH), have shown encouraging outcomes (AUC > 0.91) (46, 47).
Several systems for predicting prognosis, recurrence, and surveillance of thyroid cancers based on T, N, and M have demonstrated significantly better accuracy than the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. Most are trained on extensive demographic and clinicopathological information from public cancer registries (48, 49). Furthermore, the inclusion of molecular marker data may enhance the accuracy of these tools. For example, a study using network analysis and an ML model evaluated the association of 12 genes with the risk of LN metastasis in thyroid cancer, identifying the ERBB3 gene as both a diagnostic marker (AUC = 0.89) and a predictor of LN involvement (AUC = 0.75) (50). Another ML algorithm discovered three molecular subtypes by examining 82 genes associated with higher recurrence rates, with one subtype linked to a higher rate of RAS mutations and a lower rate of BRAFV600E mutations (51).
3.3. Treatment
3.3.1. Artificial Intelligence Application in Improving Surgical Procedures
The management of thyroid nodules involves both surgical and conservative approaches (7). Artificial intelligence can help enhance surgical procedures and outcomes. Several studies have evaluated intraoperative AI applications to improve surgical techniques and prevent common thyroidectomy complications, such as postoperative hypocalcemia within the first 30 days, damage to the recurrent laryngeal nerve, and cosmetic and functional difficulties from surgical scars. Shen et al. demonstrated that AI-guided ultrasonography based on the minimum variance algorithm during thoracoscopic PTC tumor resection led to better outcomes, including shorter hospitalization length, reduced blood loss, and decreased postoperative drainage volume and pain (52). DL-based models developed to identify the recurrent laryngeal nerve and parathyroid glands by analyzing endoscopic films during surgery have shown promising results in real-world settings, assisting both junior and senior surgeons (53, 54). Neural networks to forecast postoperative scar severity have also been developed, helping physicians choose more suitable strategies and aiding patients in dealing with the psychological burden of surgical scars. Kim et al. developed a CNN framework that could assess postoperative scar severity comparable to dermatologists (AUC = 0.912), though the model was trained on relatively small datasets of photographs and clinical information (55).
3.3.2. Artificial Intelligence Application in Non-surgical Treatment
Recent research has focused on using AI to improve radioactive iodine therapy (RAIT) for thyroid cancers. For example, an AI tool analyzed data from 83 adult patients with differentiated thyroid cancer and accurately prescribed RAI doses using imaging and blood testing data from fewer time points than conventional methods (56). Another study investigated the effectiveness of a logistic regression model for predicting RAIT success after thyroidectomy in low-risk PTC patients. This model, which combined post-RAIT clinical features and total body scan radiomics, showed better predictive performance than using clinical features or radiomics alone (AUC = 0.78 in the validation set compared to 0.65 and 0.69) (57). Furthermore, neural networks have been used to predict radiation exposure doses to the family members of thyroid cancer patients, helping identify high-risk individuals and improving treatment strategies (58).
Artificial intelligence also holds significant potential for identifying therapeutic targets for novel drug discovery and predicting therapeutic responses by analyzing and integrating large omics datasets. For example, AI tools designed a drug targeting Akt1 for peptide-based treatment of anaplastic thyroid cancer (59). In another investigation, AI-enabled virtual screening techniques identified promising therapeutic targets, such as Kir5.1, to improve treatment outcomes for patients with recurrent and metastatic thyroid cancer (60).
4. Conclusions
The development of AI marks the beginning of a new era in medicine. Artificial intelligence assists physicians in diagnosing, classifying, and treating thyroid nodules more accurately and efficiently, while also reducing specialists' workload. However, several limitations have hindered the widespread adoption of AI in real-world practice.
First, many studies rely on small, retrospective, single-center datasets, which often fail to represent the general population. This leads to selection bias, which can result in spectrum bias—where the performance of AI tools may not be replicable across diverse clinical settings. If AI models are trained on datasets that do not encompass the full spectrum of thyroid conditions, their generalizability becomes questionable (73). To address this, future studies should focus on collecting diverse datasets that include a wide range of thyroid disorders, including rare and atypical presentations (6). Additionally, multicenter studies with robust designs and external validation using different datasets are needed to enhance AI's reliability. Second, the inherent complexity of DL models can obscure the reasoning behind their outputs, leading to mistrust among healthcare providers and patients. To address this, the development of explainable AI (XAI) is essential, as it can provide insights into the decision-making processes of AI systems and enhance transparency (2). Third, the effective use of AI tools in medicine requires more than just technological advancements. Usability is a critical factor, and AI tools must be seamlessly integrated into current workflows. A lack of usability can cause clinicians to resist these innovations, limiting their potential advantages. Comprehensive training programs for healthcare professionals are essential, focusing on the proper use of AI tools while emphasizing the importance of critical thinking and clinical judgment in interpreting AI results. Inadequate training can lead to the incorrect utilization of these technologies, potentially increasing diagnostic errors. Finally, ensuring equal access to AI technologies and addressing the ethical implications of AI in healthcare is crucial (74). With sufficient financial support and careful planning, the substantial capabilities of AI can be harnessed reliably across the globe.