1. Background
Gallstones are rare in children, and many cases are asymptomatic. The diagnosis of gallstones in children often occurs incidentally during radiological procedures, particularly abdominal ultrasounds. The age at which gallstones appear in children is influenced by factors such as individual cases and environmental conditions, and it is often underestimated due to the lack of symptomatic manifestations. Reported prevalence rates in children vary between 0.13% and 2% (1-3).
The causes of gallstones in children include a variety of common causes and risk factors. Certain comorbidities, such as cystic fibrosis and hemolytic disorders, predispose children to gallstones. Additional factors that increase the risk of gallstones include obesity, dyslipidemia, type 2 diabetes, and hyperinsulinemia. Furthermore, genetic predispositions and specific medications can contribute to gallstone formation. Gallstones in children typically present with non-specific abdominal symptoms, and approximately 10 - 20% of affected children require cholecystectomy (3, 4). Cholecystectomy is the primary treatment for symptomatic gallstones, although it is not common in children (5-7). The frequency of cholecystectomy in children has been increasing in recent decades (8, 9). Limited studies on pediatric cholecystectomy indicate that the most common cause for this procedure is biliary dyskinesia (5, 10, 11). Factors contributing to the increased prevalence of gallstones include obesity, dyslipidemia, type 2 diabetes, and hyperinsulinemia (8, 12, 13).
Despite the rising rate of cholecystectomy in pediatric patients, there is insufficient information regarding changes in liver function tests (LFTs) within this population. Understanding these changes is crucial for effective postoperative care and monitoring.
2. Objectives
This study was conducted to investigate changes in laboratory enzyme tests following cholecystectomy in children with gallstones. By addressing this knowledge gap, the research aims to inform clinical practice, enhance postoperative guidelines, and reduce unnecessary testing, ultimately benefiting both physicians and patients.
3. Methods
This cross-sectional retrospective study was conducted from 2012 to 2021 on all children who underwent cholecystectomy at Mofid Children’s Hospital.
3.1. Inclusion and Exclusion Criteria
The inclusion criteria encompassed individuals under 18 years of age who had undergone a cholecystectomy. The exclusion criteria included cholecystectomies performed for reasons other than gallstones and patients with underlying diseases that could affect liver enzymes, such as cystic fibrosis and Wilson’s disease, which can cause gallstones and elevated liver enzyme levels. Additionally, patients with non-gallstone-related conditions and other pathologies were excluded. To control for confounding factors, comprehensive medical histories were collected, including details of medications that could affect liver enzymes, such as ceftriaxone use during infancy.
Data extracted from the patients’ hospital records included demographic information, symptoms, drug history (particularly ceftriaxone use during infancy), and laboratory test results. Patients presenting with abdominal pain, fever, and jaundice were assessed using ultrasound imaging of the abdomen, liver, and bile ducts. Gallstones were identified through these ultrasound examinations. Following surgical consultations, some patients were selected as candidates for cholecystectomy. Only those who underwent cholecystectomy were enrolled in the study. The cholecystectomy procedures were performed either laparoscopically or through an open approach.
3.2. Timing of Postoperative Liver Function Tests
Before surgery, patients were evaluated for liver enzyme profiles. Postoperative liver enzyme tests were conducted within 24 hours after surgery and repeated at follow-up visits, typically at 1 week, 1 month, and 3 months post-surgery, to monitor any changes in liver function.
Following surgical consultations, patients presenting with abdominal pain, fever, and jaundice were assessed using ultrasound imaging of the abdomen, liver, and bile ducts. Gallstones were identified through these examinations. Some of these patients were selected as candidates for cholecystectomy. Only those who underwent cholecystectomy were included in the study, with procedures performed either laparoscopically or via an open approach. Preoperative liver enzyme profiles were evaluated and, if necessary, repeated post-cholecystectomy. Additionally, the gallbladder was examined post-surgery by a pathologist. It is noteworthy that all patients included in this study were assessed for the presence of residual lesions and the need for re-surgery, and none required re-surgery.
3.3. Statistical Analysis
Descriptive statistics, including mean, standard deviation, median, range, frequency, and percentage, were used to analyze the data. The normality of the distribution of the examined variables was assessed using the Kolmogorov Smirnov test, which indicated that the data were not normally distributed (P < 0.05). Our findings were further supported by visual assessments using Q-Q plots to illustrate the distribution characteristics of the data. Since the distributions of pre- aspartate aminotransferase (AST) and pre-alanine aminotransferase (ALT) values were non-normal, we employed non-parametric statistical methods for our analyses. Specifically, the Wilcoxon signed-rank test was used to compare pre- and post-intervention measurements, as this test is appropriate for paired samples when normality assumptions are violated. The normality test results confirmed that the data were non-normally distributed, which justified the use of the non-parametric Wilcoxon signed-rank test for our analyses.
4. Results
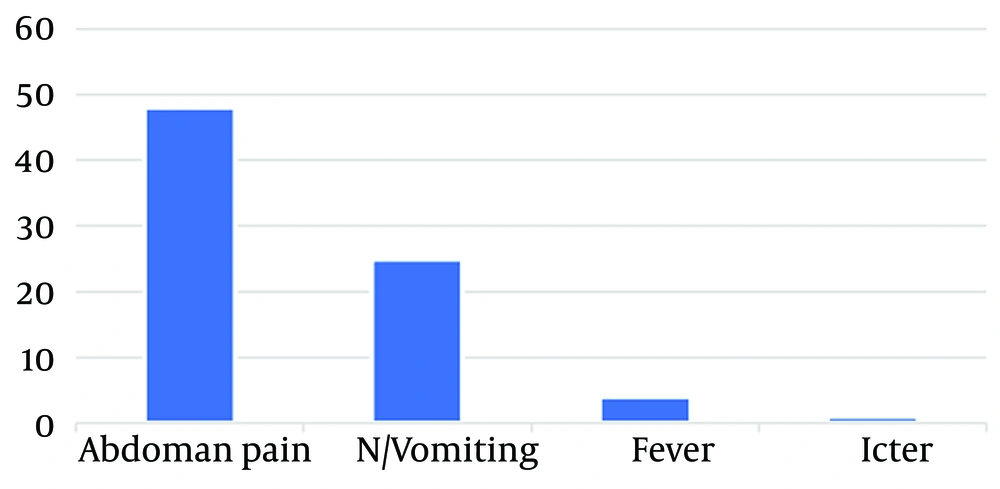
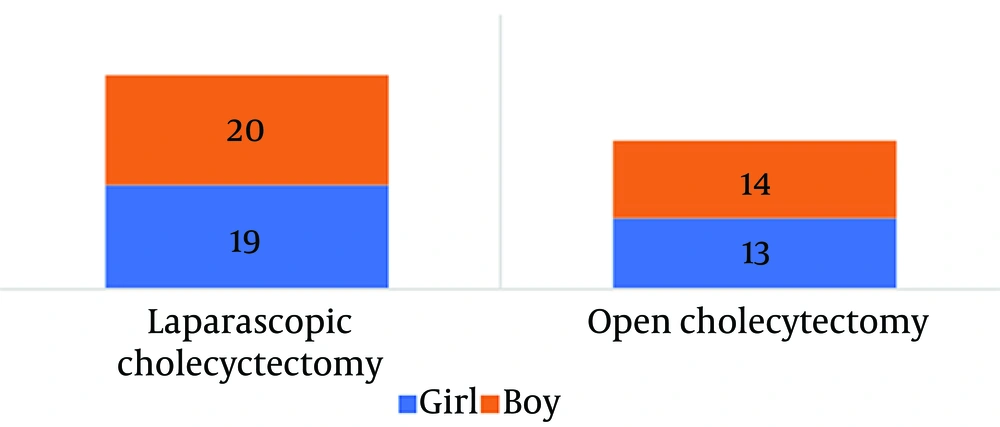
A total of 66 patients were included in this study, with 32 patients (48.48%) being boys and 34 patients (51.52%) being girls. Among them, 27 patients (40.90%) underwent open cholecystectomy, and 39 patients (59.1%) underwent laparoscopic cholecystectomy. The most common clinical symptom among the patients was abdominal pain (72.7%), and 37.8% of patients also experienced vomiting. The mean age of the patients was 2.7 ± 0.65 years. The clinical data of the patients are presented in Figures 1 and 2.
The levels of liver enzymes, including AST, ALT, and alkaline phosphatase (ALP), before and after surgery are documented in Table 1. The reductions in AST and ALT levels after cholecystectomy did not show statistical significance; the P-value for AST levels was 0.65, and for ALT levels, the P-value was 0.36. However, there was a significant decrease in ALP levels after the intervention (P-value = 0.003).
| Variables | Median | (Q1 - Q3) a, b | P-Value |
|---|---|---|---|
| Comparison of AST before and after | |||
| Pair 1 | 0.65 | ||
| Pre-AST | 69.4 | (28 - 71.5) | |
| Post-AST | 48.1 | (34.5 - 65.5) | |
| Comparison of ALT levels before and after | |||
| Pair 1 | 0.36 | ||
| Pre-ALT | 87.8 | (13.75 - 95.25) | |
| Post-ALT | 38.6 | (32 - 55.25) | |
| Comparison of ALP levels before and after | |||
| Pair 1 | 0.003 | ||
| Pre-ALP | 654 | (439.25 - 719.25) | |
| Post-ALP | 373 | (237 - 544) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
a Q1: Percentiles 25.
b Q3: Percentiles 75.
None of the patients in this study exhibited any pathological findings on post-cholecystectomy ultrasound scans, such as hepatic biloma. Furthermore, no residual lesions were observed after the surgery, eliminating the need for reoperation or further evaluation. These patients did not report symptoms such as fever, abdominal distension, or jaundice after the surgery, nor did they experience or complain of any episodes of abdominal pain following the healing of the surgical wound. Post-operative ultrasound scans showed no evidence of retained stones, and none of the patients experienced surgical complications, including wound infections or complications related to anesthesia.
5. Discussion
In this study, we evaluated LFTs before and after cholecystectomy in children. The mean age of the children was 7.2 ± 2.65 years. The sample comprised 48.48% boys and 51.52% girls. Abdominal pain was the most common clinical symptom, reported in 72.7% of cases. There were no significant differences in the levels of AST and ALT before and after surgery. However, a significant difference was observed in the levels of ALP before and after cholecystectomy. No pathological evidence was found in the specimens, and there were no post-surgery complications.
Contrary to our findings, Kim et al. reported a significant increase in the levels of AST, ALT, ALP, and bilirubin after cholecystectomy in children (5). In our study, we observed an increase in the levels of ALT and AST after cholecystectomy, although this increase was not statistically significant. We also observed a significant decrease in ALP levels after cholecystectomy. The discrepancies between the two studies could be attributed to differences in sample sizes and the populations in the study. The discrepancies between the two studies could be attributed to differences in sample sizes and populations; our study was conducted on 66 Iranian children, while the study by Kim et al. was conducted on 24 Korean children (5).
In a recent study by Choudhury and Dutta, significant increases in AST and ALT within the first 24 to 48 hours post-laparoscopic cholecystectomy were reported, attributed to CO2 pneumoperitoneum. These enzyme levels typically returned to baseline within a week, consistent with our results of transient changes. While the reduction in ALP levels remained stable, the transient nature of AST and ALT elevations suggests that although they may raise initial concerns, they usually resolve without long-term implications for patients with normal preoperative liver function (14).
In a study conducted by Maleknia and Ebrahimi, liver enzymes increased significantly after cholecystectomy, but ALP did not change significantly. This increase occurred immediately after surgery and returned to previous levels in serial tests (15). Similarly, a study by Bellad and Sahu reported a significant increase in the levels of ALT, AST, ALP, and bilirubin 24 hours after cholecystectomy, with all these tests showing a downward trend in subsequent evaluations (16). The results of our study showed not only no significant increase in liver enzymes after surgery but also a significant decrease in ALP levels after the intervention. This difference could be due to the fact that liver enzyme levels in our study were not measured immediately after surgery; rather, the tests were conducted one week post-surgery. From this perspective, our results are consistent with previous studies and confirm that liver enzyme levels returned to normal following the intervention.
Singal et al. concluded that in patients undergoing laparoscopic cholecystectomy, serum bilirubin, AST, and ALT levels increased 24 hours post-surgery compared to preoperative levels and subsequently decreased 72 hours after surgery. In other words, an increase in liver enzyme levels was not observed three days post-surgery, except for ALP. Alkaline phosphatase levels exhibited a slight decrease 24 hours post-surgery and a slight increase 72 hours post-surgery (17).
These results illustrate the impact of CO2 gas used during laparoscopy on hepatocellular damage. Therefore, it is recommended to conduct baseline liver tests prior to laparoscopy in patients with suspected liver failure or underlying liver disease. However, this recommendation is less critical for open cholecystectomy in patients with suspected liver issues (18, 19).
Cholecystectomy in cases of hepatocyte damage is often accompanied by an increase in liver enzymes. In laparoscopic cholecystectomy, compared to open cholecystectomy, a significant increase in liver enzymes can be observed, likely due to increased pneumoperitoneal pressure from the use of carbon dioxide gas during laparoscopy. However, a study by Singh et al., comparing changes in liver enzymes after open and laparoscopic cholecystectomy, showed similar increases in liver enzyme levels in patients undergoing open surgery and those undergoing laparoscopic cholecystectomy (20). This finding suggests that differences in surgical skills and techniques in both methods may account for discrepancies in liver enzyme levels after the interventions.
According to a study by Ashraf Butt et al., ALT levels and leukocyte counts increased significantly after laparoscopic cholecystectomy (21). An increase in leukocyte count is expected after invasive interventions. However, the results of our study showed that this increase was not significant, likely because blood tests were performed one week after the operation. At this point, the effect of neutrophil diapedesis on peripheral blood flow would no longer be evident. Since our patients did not experience serious complications such as sepsis or infection, significant leukocytosis was not observed.
A recent study presents findings that conflict with our results. This research reported significant increases in AST, ALT, and bilirubin levels 24 to 48 hours post-laparoscopic cholecystectomy, with P-values less than 0.05, indicating notable hepatic enzyme alterations. In contrast, our study found no statistically significant changes in AST and ALT levels post-surgery, although ALP levels decreased significantly. These discrepancies may arise from differences in sample size or methodologies used to assess liver function. The recent study highlights the possibility that laparoscopic cholecystectomy can cause clinically significant transient elevations in liver enzymes, challenging our conclusion that periodic evaluations of LFTs post-cholecystectomy are unnecessary and emphasizing the need for further investigation into these differences (15).
In a study by Akhtar-Danesh et al., involving 3519 pediatric patients who underwent cholecystectomy, the morbidity rate was 3.9%, with a lower morbidity rate observed in patients operated on due to gallstones. In contrast, our study reported no morbidity following cholecystectomy, differing slightly from the findings of Akhtar-Danesh et al. (22). This disparity may stem from differences in the ethnicity and population of the study participants, as our study was conducted in Iran, while theirs was conducted in Canada. Notably, our study spanned a duration of 10 years, during which only 66 patients underwent gallstone surgery in a referral tertiary hospital. By comparison, Akhtar-Danesh et al.'s study spanned 8 years and included 3519 pediatric cholecystectomy cases for various indications (22). This difference in the study population may also reflect the higher prevalence of gallstones in Western populations compared to Eastern populations, as noted in the literature (12).
In our study, nearly half of the patients were female. Although the gender difference was not statistically significant, a gender disparity in gallstone prevalence was observed, with a higher prevalence in females compared to males. This finding aligns with previous studies that have also reported a greater incidence of gallstones in females (23-25).
5.1. Limitations and Suggestions
Limited access to patient tests was a significant factor contributing to the small sample size in this study. Furthermore, the retrospective and cross-sectional nature of the study introduced limitations, including potential biases and reduced control over confounding variables. While the use of the Wilcoxon test for comparisons was appropriate, the small sample size may have limited the statistical power of our findings. This limitation highlights the need for caution in interpreting the results, as they may not fully represent broader patient populations.
Given these constraints, it is recommended that a prospective study be conducted with a larger patient population. Such a study should aim to compare differences in paraclinical tests between open and laparoscopic surgery, not only in the context of cholecystectomy but also across other surgical procedures where both methods are applicable.
5.2. Conclusions
Our study demonstrated that cholecystectomy does not significantly impact ALT and AST levels. However, a significant decrease was observed in ALP levels post-cholecystectomy. Based on our findings, routine monitoring of liver enzymes after cholecystectomy may not be necessary, as the lack of significant changes in ALT and AST suggests these enzymes do not require close observation post-operatively. The observed decrease in ALP levels could potentially be attributed to the resolution of the liver’s cholestatic condition following the procedure. We recommend that clinicians consider these findings when determining post-operative monitoring protocols, as unnecessary testing could increase healthcare costs without providing additional clinical benefits.