1. Introduction
Digoxin, also known as Digitalis, is a commonly used medication for the treatment of various cardiac conditions, including atrial fibrillation and heart failure. Although digoxin has not shown a significant impact on overall mortality, it has been demonstrated to reduce hospital admissions and improve patients' well-being (1, 2).
Digoxin primarily inhibits the Na⁺/K⁺-ATPase enzyme on myocardial cell membranes, which increases myocardial contractility and enhances cardiac function in patients with heart failure (3). Additionally, digoxin enhances vagal tone, leading to a slower ventricular contraction rate, which is beneficial in managing arrhythmias such as atrial fibrillation and atrial flutter (4, 5). Furthermore, digoxin potentially reduces the refractory period of the atria, increasing the atrial rate while slowing ventricular conduction (6). In summary, digoxin exerts positive inotropic effects, which can be advantageous for heart failure patients, while also reducing neuroendocrine activity (7).
Digoxin has a narrow therapeutic window, and in clinical practice, low doses are typically administered to minimize the risk of toxicity. However, careful monitoring is essential to prevent life-threatening complications due to digoxin toxicity (8).
The positive inotropic effects of digoxin occur within serum digoxin levels of 0.7 - 1.2 ng/mL, while higher concentrations may induce arrhythmias. Toxicity is most likely to occur at serum digoxin levels exceeding 2.0 ng/mL and is almost certain at levels above 3.0 ng/mL. Moreover, metabolic abnormalities such as hypokalemia, hypomagnesemia, and hypercalcemia can increase the likelihood of digoxin toxicity, even when serum digoxin levels remain within the therapeutic range. Additionally, impaired renal clearance due to underlying patient conditions can lead to elevated serum digoxin levels and an increased risk of toxicity (9).
Digoxin toxicity often presents with nonspecific symptoms, including lethargy, confusion, and gastrointestinal manifestations such as nausea, vomiting, diarrhea, and abdominal pain. Visual disturbances, including blurred vision, yellow vision, halos, and scotomas, may also occur, although they are rare in clinical practice (10-12).
Cardiac arrhythmias are a hallmark of digoxin toxicity and represent the primary cause of mortality in affected patients. Sinus bradycardia, atrioventricular block, and ventricular ectopy are common arrhythmias associated with digoxin toxicity. However, atrial and junctional tachycardia with atrioventricular block are the most characteristic findings, as tachyarrhythmia occurs simultaneously with the suppression of the sinus or atrioventricular node. In severe cases, ventricular tachycardia or fibrillation may develop (13).
There is no standardized evidence-based guideline for the management of mild to moderate digoxin toxicity. However, in severe cases, hospital admission and the administration of digoxin immune Fab, also known as digoxin-specific antibody antigen-binding fragments (DSFab), are recommended. Digoxin-specific antibodies bind to digoxin, forming molecular complexes that are subsequently excreted via urine, making DSFab the preferred antidote for digoxin toxicity (13).
Digoxin-specific antibody fragment indications include:
- Ventricular arrhythmias
- High-grade heart blocks
- Hypotension
- Symptomatic bradycardia
- Potassium levels greater than 5 mEq/L in acute overdose
- Acute ingestion of more than 10 mg in an adult or more than 4 mg in a child
- Digoxin concentration exceeding 15 ng/mL at any time
- Digoxin concentration greater than 10 ng/mL measured six hours post-ingestion
The empiric DSFab dosage is 10 to 20 vials for an acute overdose and 3 to 6 vials for chronic toxicities (14). However, contemporary sources present some disagreements regarding DSFab indications, mainly due to cost-benefit considerations, as each vial costs approximately $1,000. This economic factor remains a key point of debate (13).
Digoxin-specific antibody fragments are not readily available in pharmaceutical markets in Iran, and most patients are instead managed with conventional treatments (15, 16). This significant limitation in DSFab access worldwide, particularly in developing countries, underscores the necessity of alternative treatment strategies when managing severe cases of digoxin toxicity.
Here, we present a case of severe digoxin toxicity resulting from an acute overdose in a suicide attempt, which was successfully treated without DSFab administration.
2. Case Presentation
A 45-year-old Iranian female patient diagnosed with multiple drug poisoning, presenting with an altered mental status to the level of deep coma, was admitted to Ayatollah Taleghani Hospital in Urmia, Iran. In a suicide attempt motivated by her depressive state, she had ingested multiple drugs, including 50 tablets of 0.25 mg digoxin, 50 tablets of 2 mg clonazepam, 100 tablets of 20 mg propranolol, and 80 tablets of 350 mg acetaminophen, approximately 4 to 6 hours before hospital admission.
Her past medical history included major depressive disorder and hyperthyroidism, for which she was receiving treatment with fluoxetine, clonazepam, and propranolol at the time of admission. She had previously been admitted to the hospital multiple times due to suicide attempts involving multiple drug poisoning, with one incident occurring 40 days prior to this admission and another approximately eight months earlier.
Upon admission, the patient’s vital signs were as follows: Blood pressure of 170/110 mmHg, pulse rate of 70 beats/min, body temperature of 36°C, respiratory rate of 22 breaths/min, oxygen saturation of 88%, and a Glasgow Coma Scale (GCS) score of 8/15. Both pupils were dilated and non-responsive to light stimulation.
Based on the patient’s medical history, presenting symptoms, clinical signs, and laboratory evaluations, she was diagnosed with digoxin toxicity, and treatment was initiated immediately. The initial goal was to stabilize the patient’s cardiovascular status. In the emergency room (ER), an intravenous (IV) line was established, and fluid replacement therapy was initiated. Due to her altered mental status and respiratory distress, the patient was intubated for mechanical ventilation. Cardiac monitoring and electrocardiography (ECG) were performed, and an urgent cardiology consultation was requested.
For gastric support, a vial of 40 mg IV pantoprazole was administered and scheduled to be repeated daily. A nasogastric tube was inserted for gastric lavage and administration of activated charcoal (1 g/kg) with sorbitol (1 g/kg) in 150 cc of water, repeated every four hours until laxation. Due to the ingestion of multiple acetaminophen tablets, a 21-hour protocol of N-acetylcysteine (NAC) was initiated for liver support. Blood and urine samples were collected for hematology, biochemistry, serology, and toxicology evaluations.
Laboratory tests performed on the day of admission, approximately 4 to 6 hours after ingestion of the reported drugs, revealed the following results: White blood cell count of 21,900/mm3 (90% neutrophils, 8% lymphocytes, and 2% mixed cells), red blood cell count of 5.89 million/mm3, hemoglobin level of 18.1 g/dL, hematocrit level of 56.6%, mean corpuscular volume of 96.1 fL, mean corpuscular hemoglobin of 30.73 pg, mean corpuscular hemoglobin concentration of 31.98%, platelet count of 381,000/mm3, prothrombin time of 14.5 seconds, and partial thromboplastin time of 27 seconds.
Other laboratory findings included a blood sugar level of 188 mg/dL, urea level of 47 mg/dL, creatinine level of 1.4 mg/dL, lactate dehydrogenase level of 531 IU/L, creatine kinase-myoglobin binding level of 65 U/L, and a negative troponin I result. Electrolyte levels were as follows: Sodium 140 mEq/L, potassium 5.5 mEq/L, magnesium 1.8 mg/dL, calcium 8 mg/dL, and phosphorus 4.7 mg/dL. Venous blood gas analysis showed a pH of 7.160, CO2 pressure of 48.4 mmHg, O2 pressure of 43.5 mmHg, and bicarbonate level of 17.4 mmol/L. The C-reactive protein level was 25.8 mg/L.
Urine toxicology screening tested positive for barbiturates, morphine, and tramadol. The serum digoxin concentration was measured at 5.6 ng/mL.
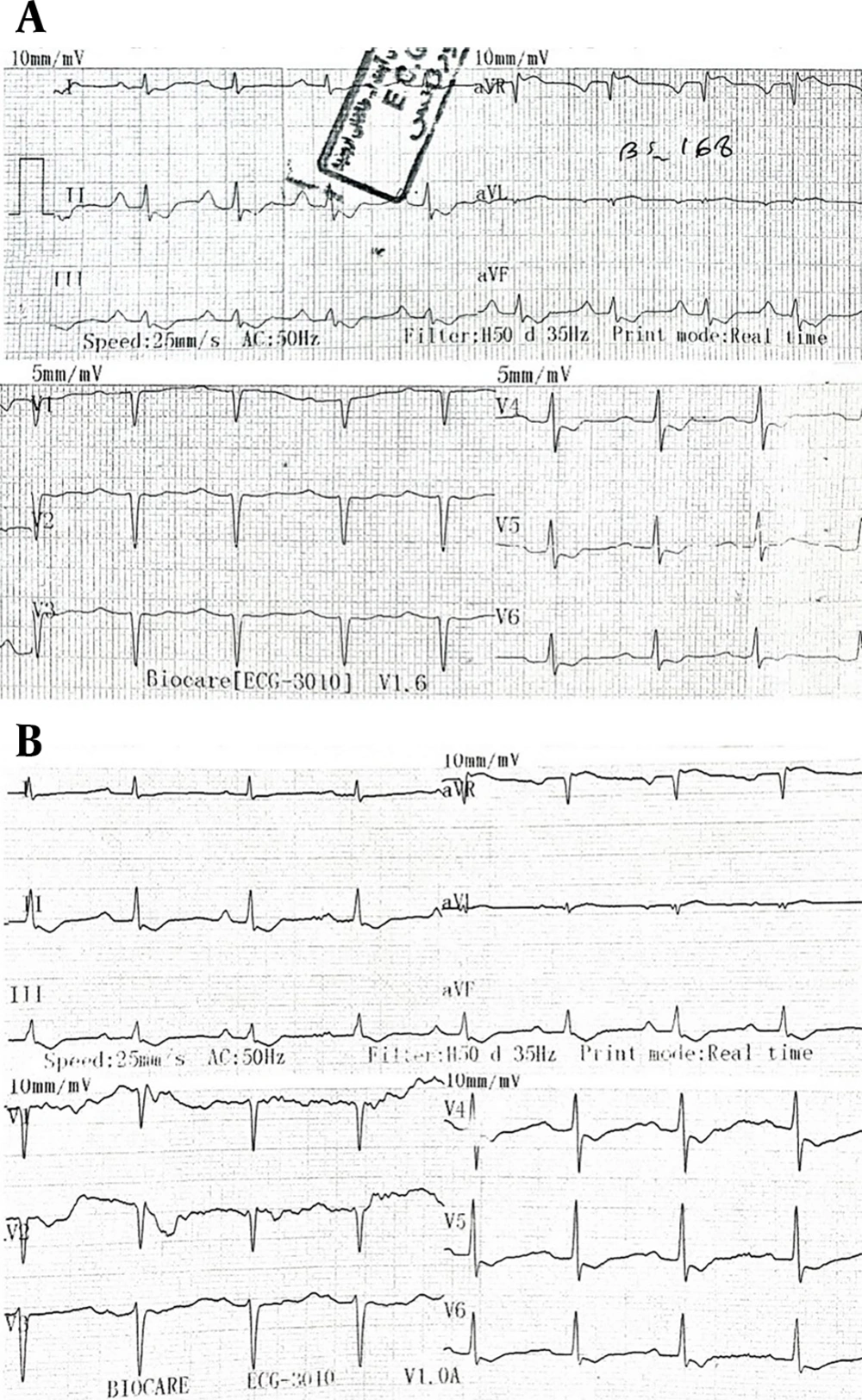
The ECG taken upon admission showed sinus tachycardia with a heart rate of 105 bpm, which the cardiology consultant suggested was likely unrelated to the acute multiple drug poisoning (Figure 1A). Approximately five hours later, ECG findings revealed ST depression in multiple leads, which, according to the cardiology consultation, was most likely secondary to digoxin effects (Figure 1B). However, there were no signs of bradycardia or any specific arrhythmias.
A, the earliest electrocardiography (ECG) performed in the emergency room (ER) shows sinus tachycardia with a heart rate of 105 as well as the digoxin effect with a curved ST segment depression in various leads; B, the ECG performed on the day of admission after the patient was admitted to the intensive care unit (ICU) about 5 hours after referring to the ER also shows the digoxin effect with curved ST segment depression in various leads.
The patient was then admitted to the intensive care unit (ICU), where treatment continued, including mechanical ventilation, fluid replacement therapy, administration of laxatives, and gastric lavage. She was closely monitored for cardiac function, electrolyte imbalances (particularly potassium levels), and acid-base disturbances. Serial serum measurements of digoxin, potassium, and other electrolytes, as well as venous blood gas analysis, were conducted. Simultaneously, other conditions were managed, including infections, liver support, and anticoagulant therapy, among others.
This case of severe digoxin toxicity would have ideally benefited from DSFab therapy. However, due to the lack of availability of this expensive medication in the Iranian pharmaceutical market, conventional therapy was pursued instead, with close monitoring of clinical and laboratory parameters. This approach proved to be effective in the patient’s recovery.
Following stabilization, additional consultations were sought, including anesthesiology, internal medicine, psychiatry, neurology, and infectious diseases, based on the patient's medical history and presenting symptoms.
A spiral chest CT scan revealed bilateral alveolar opacifications in the upper and lower lobes, suggestive of pneumonia. The infectious disease consultant recommended IV antibiotic therapy. Brain imaging and cervical vessel color Doppler ultrasound, performed in accordance with neurology consultation recommendations, showed no abnormalities.
The patient’s condition progressively improved with conventional treatment. Given that cardiac disturbances, particularly arrhythmias, are the most critical complications of digoxin toxicity, two key parameters — serum digoxin concentration and serum potassium levels — were closely monitored. Serum digoxin concentration gradually decreased, reaching 0.78 ng/mL on the seventh day of admission, while potassium levels remained within a controlled range throughout hospitalization (Table 1).
| Day of Admission | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum digoxin concentration (ng/mL) | 5.60 | 4.59 | 2.86 | 0.96 | 1.84 | 1.20 | 0.78 | - | - | - | - |
| Serum potassium level (mEq/L) | 5.5 | 3.6 | 3.0 | 3.0 | 3.1 | 3.4 | 4.0 | 3.3 | 4.1 | 4.5 | 4.2 |
This outcome was considered highly favorable, as it demonstrated a similar trend to that seen with DSFab treatment, which pharmacologically reduces serum digoxin concentration by molecular binding while stabilizing potassium levels, thereby improving myocardial contractility. Given the absence of DSFab, these parameters were even more crucial for ensuring the patient’s safety.
The patient was extubated nine days after admission and was transferred from the ICU to the toxicology ward on the 13th day. She was discharged in stable condition after 19 days of hospitalization, with recommendations for psychiatric and internal medicine follow-up.
3. Discussion
Digoxin toxicity most commonly presents with gastrointestinal symptoms such as nausea and vomiting (14). Cardiac arrhythmias are also a frequent manifestation of digoxin overdose (13). However, our patient did not exhibit typical signs and symptoms of digoxin toxicity, nor did she experience any specific or severe cardiac arrhythmias. Although ST-segment depressions were observed on ECG, they were likely due to the digoxin effect rather than an acute ischemic event (17).
Regarding the indications for DSFab administration, our patient met two criteria: Potassium levels greater than 5 mEq/L in an acute overdose (her potassium level was 5.5 mEq/L upon admission) and acute ingestion of more than 10 mg of digoxin, as she had ingested 50 tablets of 0.25 mg digoxin, totaling 12.5 mg (14). Despite meeting these criteria, she did not receive DSFab due to its unavailability in the pharmaceutical market and was instead treated conventionally based on her clinical presentation and underlying conditions (15, 16).
Hyperkalemia is a common finding in digoxin toxicity and is considered a marker of severity (14, 17). Although our patient initially presented with a potassium level of 5.5 mEq/L, her electrolyte imbalance was effectively managed with fluid replacement therapy, and potassium levels, along with other electrolytes, were closely monitored.
Cardiac monitoring and serial ECG evaluations were performed due to the patient's transient hyperkalemia, but no significant arrhythmias or abnormalities associated with electrolyte imbalances were detected.
The patient also received treatment for concurrent medical conditions, including liver support and infection management with antibiotics.
N-acetylcysteine is an FDA-approved antidote for hepatotoxic doses of acetaminophen and is most effective when administered within eight hours of ingestion (18). Our patient received NAC therapy approximately 4 to 6 hours post-ingestion, and treatment continued after her ICU admission. Additionally, 140 mg of Livergol (Goldaru Company, Iran), an extract of Silybum marianum (milk thistle), was administered, as it has been shown to have hepatoprotective effects and aid in the recovery of hepatocytes (19).
In a retrospective study by Sanaei-Zadeh et al. in Iran, evaluating the outcomes of digoxin toxicity without DSFab administration, suicidal digoxin poisoning cases responded well to conventional treatment, regardless of underlying cardiac conditions or chronic digoxin use (16). This supports the notion that, in the absence of DSFab, close monitoring and conventional therapy can still lead to favorable patient outcomes.
Likewise, in a case report by Juneja et al. in India, a patient with severe digoxin toxicity was successfully treated with resin hemoperfusion despite having serum digoxin levels as high as 12.63 ng/mL. Although DSFab was indicated, it was unavailable, and alternative treatment strategies proved effective (20).
The conventional therapy used in this case demonstrated several potential advantages over DSFab. Firstly, the financial cost of these treatments was significantly lower. Secondly, the methods employed were more accessible in various healthcare centers, including those in developing countries. Thirdly, these approaches were generally safer, with fewer risks of severe side effects compared to DSFab, which carries the potential for life-threatening complications such as severe hypokalemia.
However, the primary limitation of conventional therapy is the lack of extensive studies on its efficacy in severe digoxin toxicity cases. A more structured algorithm for managing severe cases with different medical backgrounds is necessary. Given the unavailability of DSFab in many regions, developing standardized protocols for alternative treatment methods is essential. While DSFab remains a more direct and definitive solution, acting as a molecular binder to digoxin, conventional therapy, when closely monitored, may offer a viable substitute. Our findings align with prior studies suggesting that conventional therapy can be an effective alternative in severe digoxin toxicity, provided that patients undergo continuous monitoring and supportive care (13, 16).
3.1. Conclusions
Despite not receiving DSFab, the patient demonstrated steady clinical improvement with well-managed conventional therapy, close monitoring, and supportive care.
Given the limited availability of DSFab, alternative treatment protocols show promising outcomes in the management of digoxin toxicity.
It is crucial to emphasize that throughout the patient's hospital course, electrolyte balance, cardiac monitoring, and clinical judgment played a pivotal role in ensuring a favorable outcome. Furthermore, clinical symptoms, cardiac status, and electrolyte balance appear to be more critical factors in determining the need for DSFab than the ingested dose or serum digoxin concentration alone.
Finally, further studies are needed to validate these findings in larger cohorts and establish structured protocols for DSFab alternatives to ensure optimal management of severe digoxin toxicity cases.