1. Background
Dental caries, a biofilm-associated disease, is the most common condition affecting the oral cavity (1). Streptococcus mutans, a gram-positive anaerobic bacterium, is a key contributor to dental caries (2, 3). Critical aspects of dental caries include adherence to enamel surfaces, the production of acidic metabolites, the capacity to form glycogen reserves, and the ability to produce extracellular polysaccharides (4). The production of acid by S. mutans leads to demineralization of the tooth structure, resulting in dental caries (5).
Many prophylactic agents, such as mouthwashes and toothpaste, have been introduced to prevent dental caries (6). To reduce the side effects associated with chemical agents, plant-based products have gained importance in new approaches to prevention and treatment.
Apium graveolens Linn. (celery), a member of the family Apiaceae, is recognized as a medicinal plant in traditional medicine (7, 8). Phytochemical analysis of celery has revealed the presence of phenols, flavonoids, steroids, tannins, and saponins (9, 10). Celery has demonstrated antioxidant, gastroprotective, neuroprotective, and cytotoxic properties (10, 11). Additionally, its chemical compounds exhibit analgesic, anti-inflammatory, and antimicrobial effects (11, 12).
Several studies have reported the antibacterial activity of A. graveolens. Baananou et al. demonstrated that the essential oil of A. graveolens had a strong inhibitory effect on Escherichia coli and a moderate inhibitory effect on Pseudomonas aeruginosa and Staphylococcus aureus (13). Similarly, studies by Khotimah et al. and Misic et al. showed that celery extracts exhibited relatively strong antibacterial effects against S. aureus (14, 15).
2. Objectives
Accordingly, the present study aims to evaluate the antibacterial activity of A. graveolens against S. mutans and compare the antibacterial effects of celery seed essence (essential oil) and hydroalcoholic (ethanolic) extract of celery against S. mutans in vitro. This research seeks to contribute to the development of herbal products such as toothpaste, mouthwash, and gel with antimicrobial activity targeting the primary cause of dental caries.
3. Methods
This study was approved by the Ethics Committee of Shiraz University with the code IR.SUMS.DENTAL.REC.1399.214. As this was an in vitro investigation, no humans or animals were involved.
3.1. Preparation of Plant Extract and Essence
3.1.1. Plant Extraction Preparation
Different parts of A. graveolens (celery) were purchased from the local market in Shiraz, Fars Province, Iran, in May 2021. The plant materials were identified, and a voucher number (3049-A. graveolens L.) was issued by the Shiraz School of Pharmacy.
The stems and leaves of the plant were washed thoroughly and then dried at room temperature for a week before being ground into a fine powder. Fifty grams of the plant powder were added to 1000 mL of a hydroalcoholic solution (70% ethanol). The mixture of solvent and plant powder was stirred using a magnetic stirrer device for 48 hours at room temperature to extract soluble components. The resulting extract was filtered through standard filter paper and concentrated using a rotary evaporator (EYELA-Japan). The concentrate was further processed in a vacuum centrifuge (Christ-Germany) at 48°C for 24 hours. It was then placed in a freeze dryer (Christ-Germany) for an additional 24 hours to remove any remaining solvent. The dry extract was stored in a refrigerator until further use.
3.1.2. Plant Essence (Essential Oil) Preparation
The celery seeds were purchased from the local market in Shiraz, Fars Province, Iran, in May 2021. The seeds were authenticated, and a voucher number (PM1360-Apium graveolens L.) was issued by the Shiraz School of Pharmacy.
The seeds were ground using a mill. For every 100 g of celery seeds, 1000 mL of distilled water was added, and the essence was extracted over 4 hours through hydrodistillation using a Clevenger-type apparatus. The extracted essence was stored in a freezer at -18°C until further use.
3.1.3. Preparation of Different Concentrations of the Essence and Hydroalcoholic Extract of Celery
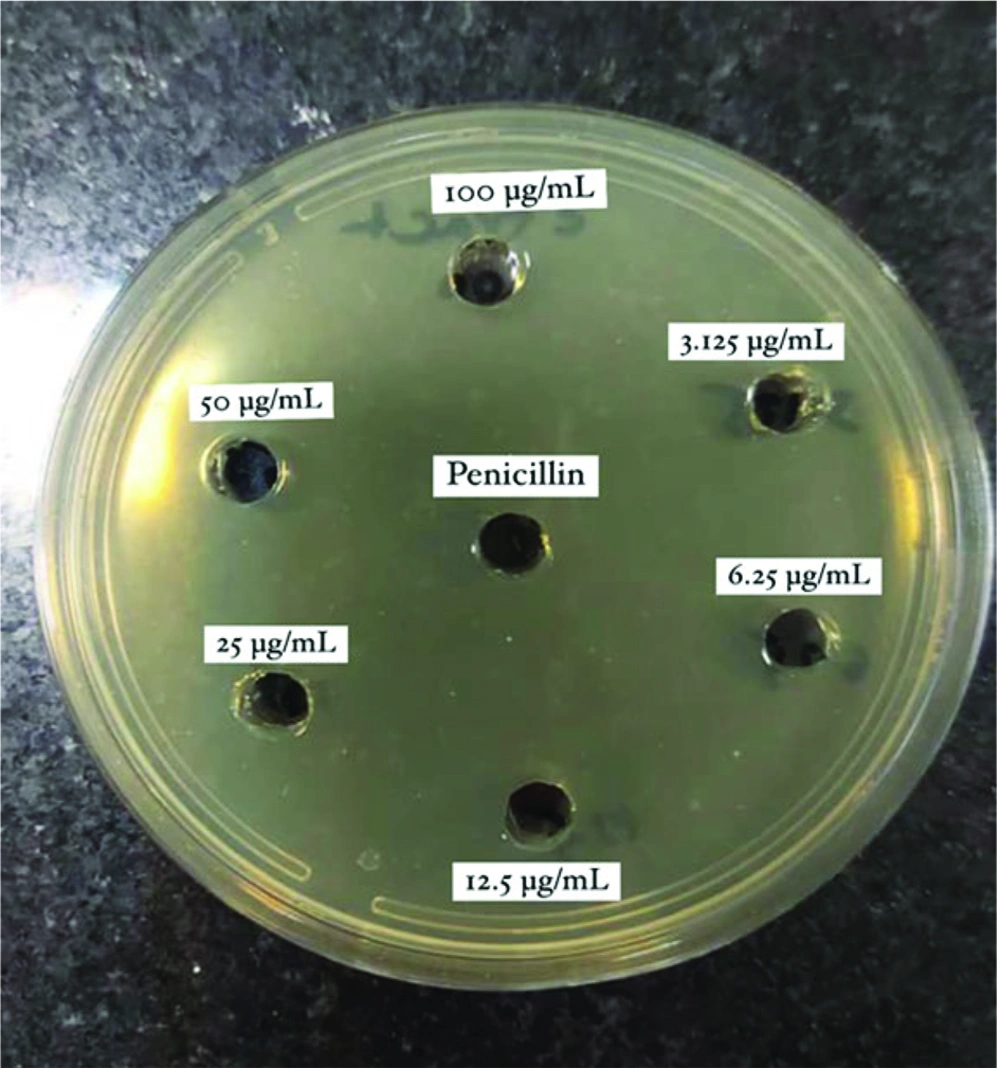
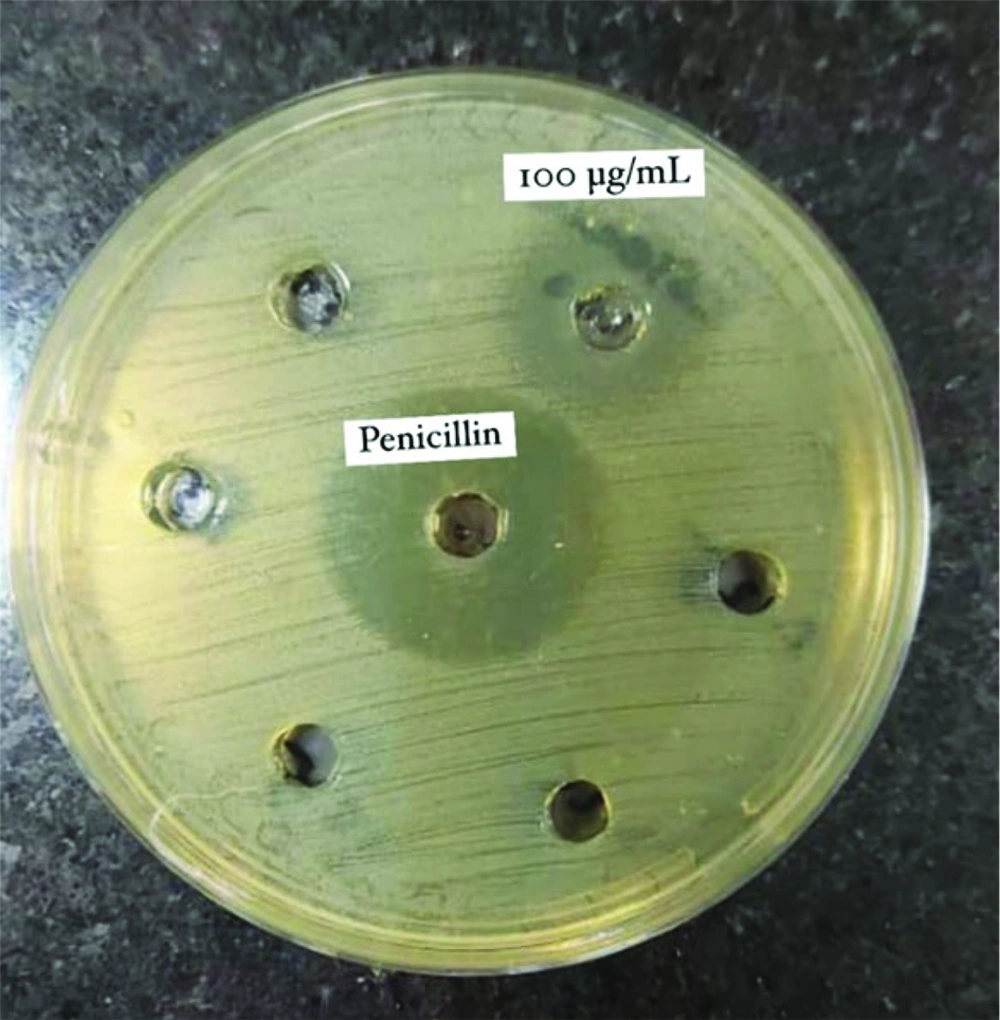
Two mg of dry celery extract and 2 mg of celery seeds essence were weighed using digital scales. The dry extract was dissolved in 20 mL of sterile water, while the essence was dissolved in 20 mL of dimethyl sulfoxide (DMSO) to achieve a concentration of 100 µg/mL for each sample. The study sample size consisted of 12 samples, divided into 6 different concentrations for each group (6 concentrations for the extract and 6 for the essential oil), along with BHI medium as the negative control and BHI medium plus bacterial suspension as the positive growth control. Additionally, penicillin was used as the positive control in the agar well diffusion assay. Finally, for both the essence and extract, 6 different concentrations were prepared using the serial dilution method: 100 µg/mL, 50 µg/mL, 25 µg/mL, 12.5 µg/mL, 6.25 µg/mL, and 3.125 µg/mL.
3.2. Antibacterial Assay
A standard strain of S. mutans (ATCC 25275) was obtained from the Department of Bacteriology and Virology at Shiraz Medical School.
3.3. Agar Well Diffusion Assay
The antibacterial activities of the hydroalcoholic extract of A. graveolens and the essence of celery seeds against S. mutans were evaluated using the agar well diffusion method. First, a fresh culture of S. mutans was prepared in a blood agar medium. A suspension with a turbidity of 0.5 McFarland (1.5 × 10⁸ CFU/mL) was then prepared in brain heart infusion (BHI) broth. A 100 µL aliquot of the S. mutans suspension was applied onto sterile Muller–Hinton agar (MHA, Merck, Germany). Wells with a diameter of 6 mm were cut into the agar using a sterile cork-borer, and each well was filled with 100 µL of different concentrations of the celery seed essence (3.125 - 100 µg/mL) and hydroalcoholic extract of celery (3.125 - 100 µg/mL). One well was filled with 5 U/mL of penicillin as a positive control.
To ensure proper diffusion of the celery seed essence and the hydroalcoholic extract of celery in the agar, the plates containing the essence were kept at room temperature for 1 hour, while the plates containing the extract were refrigerated at 5°C for 2 hours. The plates were then incubated for 24 hours at 37°C.
Triplicates were prepared for each sample. Finally, the inhibition zones were measured in millimeters.
3.4. Determination of Minimum Inhibitory Concentrations and Minimum Bactericidal Concentrations
Microtiter broth dilution assay was performed to determine the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the extract and essence. A 100 μL aliquot of a bacterial suspension with 0.5 McFarland turbidity was added to the wells of a 96-well microtiter plate. Subsequently, 100 μL of the highest concentration of each sample was added to the first well, and serial dilutions were prepared across the wells (100 - 3.125 µg/mL). One well was filled with BHI medium as a negative control, and another well was filled with BHI medium and the bacterial suspension as a growth control. Additionally, penicillin was used as a positive control.
After 24 hours of incubation at 37°C, the lowest concentration of the samples that showed no visible signs of bacterial growth was recorded as the MIC.
To determine the MBC, 5 μL of the contents from wells that showed no signs of bacterial growth were cultured on Muller–Hinton agar. The plates were then incubated at 37°C for 18 - 24 hours. The concentration of the sample that produced fewer than 10 colonies, as evaluated by a colony counter (QUEBEC), was considered the MBC value.
Each procedure was repeated at least three times to ensure accuracy and reproducibility.
3.5. Statistical Analysis
SPSS version 22 was used for data analysis. Descriptive data were analyzed using means and standard deviations. Comparisons of means were conducted using the Kruskal-Wallis H test, and pairwise comparisons were further evaluated with Dunn’s Post-hoc test.
4. Results
In this study, the antibacterial activity of the hydroalcoholic extract of celery (leaves and stems) and the essence of celery seeds against S. mutans was assessed at six different concentrations, as shown in Figures 1 and 2.
The hydroalcoholic extract demonstrated consistent antibacterial activity across all evaluated concentrations, while the essence of A. graveolens seeds inhibited S. mutans proliferation only at a concentration of 100 µg/mL, producing an inhibition zone of 21 mm (Table 1).
| Samples and Concentrations (µg/mL) | Mean ± SD (mm) |
|---|---|
| Hydroalcoholic extract of Apium graveolens (leaves and stems) | |
| 100 | 34 ± 1 |
| 50 | 34 ± 1 |
| 25 | 34 ± 1 |
| 12.5 | 34 ± 1 |
| 6.25 | 34 ± 1 |
| 3.125 | 34 ± 1 |
| Essence of Apium graveolens (seeds) | |
| 100 | 21 |
| 50 | 0 |
| 25 | 0 |
| 12.5 | 0 |
| 6.25 | 0 |
| 3.125 | 0 |
| 5 | 28 |
Mean Diameter of Zone of Inhibition of Streptococcus mutans, in Different Concentrations of Hydroalcoholic Extract and Essence of Apium graveolens
The MIC and MBC values for the hydroalcoholic extract of celery (leaves and stems) and the essence of celery seeds are presented in Table 2.
| Samples | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| Hydroalcoholic extract of Apium graveolens (leaves and stems) | 3.9 ± 1.56 | 3.9 ± 1.56 |
| Essence of Apium graveolens (seeds) | 100 | 100 |
| Penicillin | ≤ 0.12 | ≤ 0.12 |
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Hydroalcoholic Extract and Essence of Apium graveolens Against Streptococcus mutansa
The MIC and MBC for the hydroalcoholic extract of celery (leaves and stems) were determined to be 3.9 ± 1.56 µg/mL, while the MIC and MBC of the essence (celery seeds) were 100 µg/mL. The MIC and MBC values of the celery ethanolic extract were lower than those of the celery essence.
According to Table 3, using the Kruskal-Wallis H test and Dunn’s Post-hoc test for pairwise comparisons, the MIC and MBC values of the three evaluated groups (hydroalcoholic extract of celery leaves and stems, essence of celery seeds, and penicillin) were significantly different (P-value = 0.005).
| Group | MIC Median (IQR) | MBC Median (IQR) |
|---|---|---|
| Hydroalcoholic extract of Apium graveolens (leaves and stems) | 3.125 (2.34) ABC | 3.125 (2.34) ABC |
| Essence of Apium graveolens (seeds) | 100 (0) B | 100 (0) B |
| Penicillin | 0.12 (0) C | 0.12 (0) C |
| P-value b | 0.005 | 0.005 |
The Comparison of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Between Different Groups a
In pairwise comparisons of MIC and MBC between different groups, no statistically significant difference was found between the hydroalcoholic extract and penicillin, nor between the hydroalcoholic extract and the essence. However, penicillin exhibited significantly lower MIC and MBC values compared to the celery essence (P-value = 0.003).
5. Discussion
To the best of our knowledge, previous studies have confirmed the antibacterial effect of celery, though varying MIC and MBC values have been reported (16-18). However, there are limited studies on the antibacterial effect of celery extract against S. mutans.
In one study, a toothpaste was formulated with three different concentrations (6.25%, 12.5%, and 25%) of celery leaf ethanolic extract. The largest zone of inhibition against S. mutans was observed at a concentration of 12.5% (18.3 ± 0.57 mm) (16).
According to Nair et al., who compared five different concentrations of ethanolic extract of celery leaves against S. mutans, the most inhibitory effect was observed at a concentration of 100 µg/mL (17).
In another study investigating the antibacterial effect of celery extract against S. mutans, the MIC was reported as 3.125%, but the extract exhibited no bactericidal effect (18).
In line with the results of the studies mentioned above, the findings of the present study confirmed the antibacterial effect of the hydroalcoholic extract of celery (leaves and stems) and the essence of celery seeds. The essence of celery seeds at a concentration of 100 µg/mL exhibited the highest antibacterial effect, creating an inhibition zone of 21 mm. The MIC and MBC of this concentration of celery seeds essence were both 100 µg/mL against S. mutans.
The MIC and MBC of the hydroalcoholic extract of celery (leaves and stems) against S. mutans in this study were 3.9 ± 1.56 µg/mL, which was significantly lower than the MIC value reported in Nair et al.'s study (100 µg/mL) (17). In this study, both leaves and stems of celery were used to prepare the hydroalcoholic extract, whereas Nair et al. used only celery leaves. Additionally, different celery species and variations in geographic regions may account for differences in antibacterial contents, leading to varying MIC and MBC values (17).
The antibacterial efficacy of A. graveolens against different bacterial species has also been assessed in other studies (10, 15, 19). Uddin et al. reported MIC values of 1.11 ± 0.5 µg/mL and 0.5 ± 0.2 µg/mL for methanolic and ethanolic extracts of A. graveolens against S. aureus, respectively (10). Another study examined the antibacterial effect of celery seed essential oil of Indian origin against S. aureus, with a zone of inhibition measuring 17.1 ± 0.76 mm (19). According to Misic et al., celery seed extract exhibited significant inhibitory effects on Bacillus, Listeria, and S. aureus strains, with MIC values ranging from 160 to 640 µg/mL (15).
According to the results of the present study and most previous evaluations, the antibacterial effect of Apium graveolens has been confirmed. The extent of this property depends on several factors, including the geographic region of the plant, the season of harvest, soil composition, the methodology of laboratory assessment, the type of solvent used, and the concentration of extract and essence. Assessing the chemical composition of A. graveolens species and identifying the most effective components for antibacterial properties can explain differences in the antimicrobial effects of these herbal products.
Apium graveolens contains flavonoids, tannins, saponins, and steroids. The essential oil of celery seeds includes compounds such as limonene, selinene, furocoumarin, and furocoumarin glycosides, along with flavonoids. Additionally, the presence of flavonoid apigenin, as well as vitamins A and C, has been confirmed (20). Phenols are present in celery leaves and stems. Components such as apigenin in celery leaves include flavonoids, luteolin, chrysoeriol 7-glucosides, furanocoumarins (psoralen, bergapten, xanthotoxin), and isopimpinellin (9).
Celery has demonstrated antibacterial effects against both gram-positive and gram-negative bacteria (21). Phytochemical agents in celery can enhance antibacterial activity either independently or in combination with antibiotics (22). Compounds such as flavonoids, alkaloids, and saponins are known to exhibit antibacterial effects (21). These effects may involve binding of free hydroxyl groups, limonene, or β-selinene to carbohydrates and proteins in the bacterial cell wall. The lipophilic nature of these compounds, present in A. graveolens extract, contributes to their antibacterial activities, which may occur through enzyme inhibition or disruption of energy pathways by their accumulation in bacterial membranes (12, 19).
Flavonoids, recognized for their antimicrobial properties, can exert antibacterial effects through mechanisms such as inhibiting energy metabolism and nucleic acid synthesis (23). Flavone, one of the flavonoids found in celery, has been reported to inhibit helicase, a crucial enzyme in the bacterial DNA replication process, thereby disrupting cell division and bacterial reproduction. Additionally, flavone can inhibit microbial adhesion and growth by forming complexes with components of the bacterial cell wall (24-26).
Saponins also exhibit antibacterial properties by disrupting and increasing the permeability of bacterial cell membranes. Antibacterial components like terpenoids, alkaloids, and phenolic compounds can induce cell death or inhibit enzyme activities by interacting with bacterial cell membrane proteins and enzymes (7, 21, 23).
Some prior studies did not clearly report the evaluated concentrations of celery used in their research, nor did they consistently describe the extraction methods employed. These factors complicate direct comparisons between studies.
One limitation of this study was the use of a standard strain of S. mutans instead of strains cultivated from intraoral biofilms. Future studies are recommended to evaluate the antibiofilm effects of A. graveolens against S. mutans and other oral pathogenic bacteria.
5.1. Conclusions
Ethanolic extract of celery stems and leaves and celery seeds essence both exhibited significant antibacterial properties against S. mutans. The MIC and MBC of the hydroalcoholic extract of celery (leaves and stems) were 3.9 ± 1.56 µg/mL, while the MIC and MBC of celery seeds essence were 100 µg/mL. The ethanolic extract of celery demonstrated a stronger antibacterial effect against S. mutans compared to celery seeds essence.
The results of the antibacterial assays in this study provide valuable insights that could contribute to the development of effective products for inhibiting the progression of dental caries and for pharmaceutical applications. However, further research is recommended to validate these findings and explore their practical applications.