1. Background
Immunodeficiency disorders, whether acquired or inherited, significantly impact immune pathways (1). Primary immunodeficiencies (PIDs) arise from genetic anomalies, immunosuppressive treatment, and acquired immunodeficiency syndrome (AIDS), all of which compromise immune function (2). The human gastrointestinal (GI) tract hosts the largest microbiota burden, containing trillions of microorganisms, including approximately 4,000 commensal species (3, 4). The gut mucosa and gut-associated lymphoid tissue (GALT), along with the microbiota, regulate immunity and defend against pathogens and food antigens (5, 6). Immunodeficiency disorders disrupt this system, allowing pathogens to breach the mucosal barrier, potentially leading to infections or virus-induced malignancies (2). Patients with PID frequently present with GI symptoms, including chronic diarrhea, low body mass, abdominal pain, and failure to thrive (FTT). These symptoms can result in inflammation, infection, autoimmunity, or malignancy (7).
2. Objectives
Despite the frequency of these manifestations, there is a significant knowledge gap in their understanding and management. This study was conducted to explore GI manifestations in Iranian pediatric patients with PIDs. The findings provide valuable information for formulating more effective diagnostic criteria and treatment protocols, thereby enhancing patient care and stimulating further research in pediatric immunology and gastroenterology.
3. Methods
This was a cross-sectional study conducted on children who were retrospectively evaluated for immunodeficiency disorders. Participants were selected from medical records at Mofid Children's Hospital, Tehran, Iran, from 2011 to 2022, a tertiary care hospital for children with immunodeficiency disorders. As this was a retrospective study, informed consent was not obtained from the participants.
3.1. Inclusion and Exclusion Criteria
The study included children diagnosed with an immune deficiency disorder and treated at a tertiary care hospital. Inclusion criteria were children with documented evidence of immunodeficiency, either combined immunodeficiency (CID) or severe combined immunodeficiency (SCID), who had undergone GI evaluations. Exclusion criteria included incomplete medical files, absence of GI diagnostic procedures, and underlying conditions that could confound results, such as chronic GI diseases unrelated to immunodeficiency, like celiac disease. Additional exclusion criteria included a history of immunosuppressive therapy for conditions other than immunodeficiencies, to limit the study to GI manifestations directly related to immunodeficiency disorders.
3.2. Data Collection
Data included records of age, sex, patients' medical history, and types of immunodeficiency disorders. The specific types of immunodeficiency states reviewed ranged from combined deficiencies, such as SCID, to other forms. The clinical presentation and laboratory investigations at presentation were documented.
3.3. Clinical Presentation
Clinical signs and symptoms were documented with an emphasis on GI symptoms. The most common clinical symptoms were diarrhea and vomiting. Other symptoms included nausea, fatigue, anorexia, constipation, abdominal pain, poor feeding, hematochezia, jaundice, pruritus, limb edema, splenomegaly, hepatomegaly, malabsorption, oral aphthous ulcers, anal abscess, ascites, and FTT.
3.3.1. Endoscopy
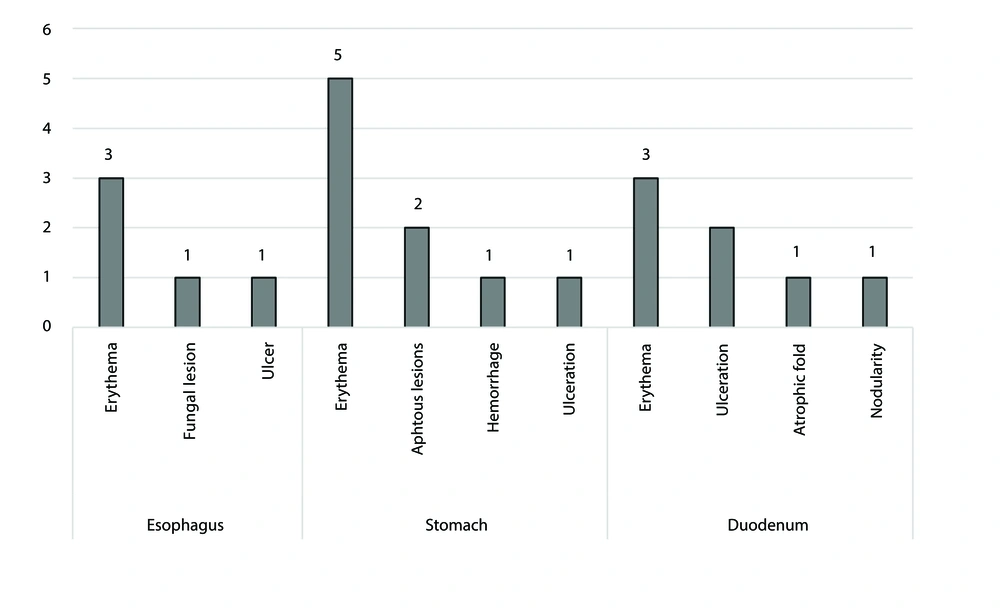
Patients underwent upper endoscopy and colonoscopy for the assessment of the GI tract. These modalities demonstrated erythema of the esophagus, stomach, and duodenum, nodularity of the colon, and erythema of the rectum.
3.3.2. Histopathological Examination
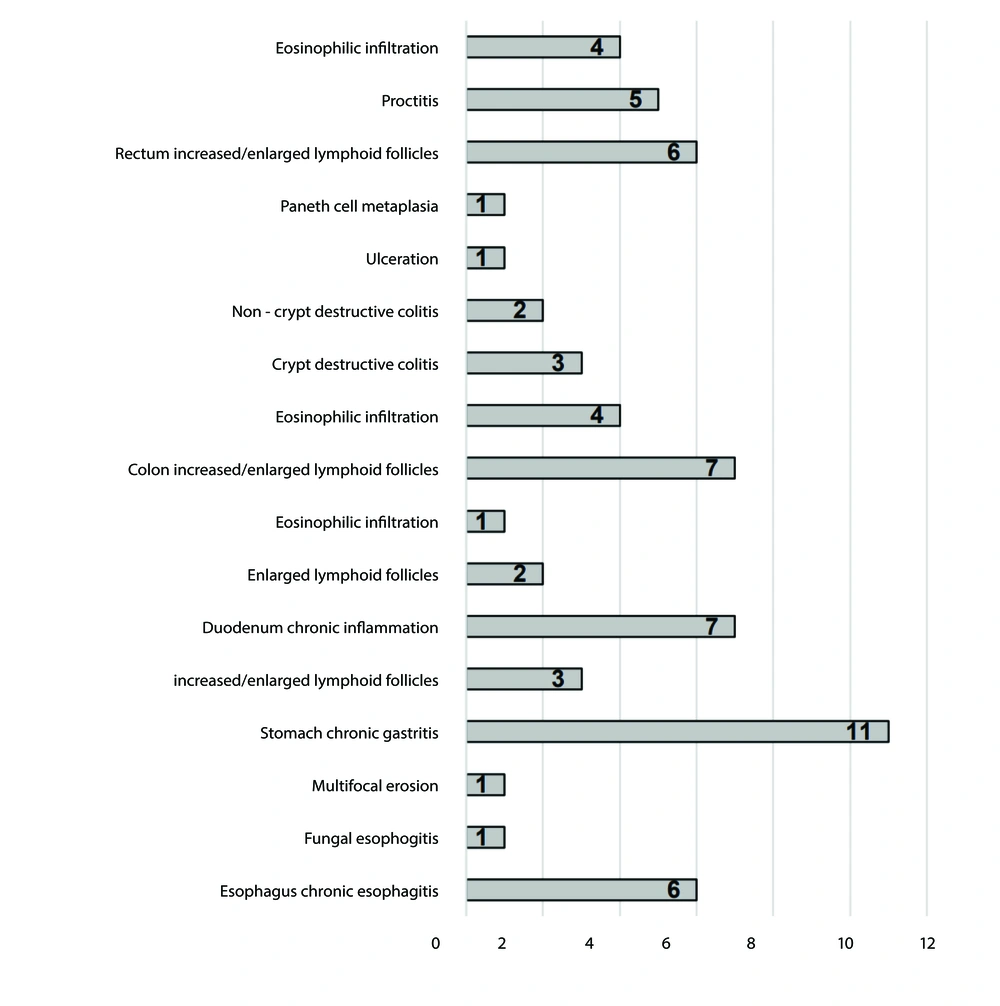
Biopsy specimens taken during endoscopy indicated various histopathological changes. Chronic esophagitis, chronic gastritis, chronic inflammatory changes in the duodenum, and increased/enlarged lymphoid follicles in the colon and rectum were frequent histopathological alterations encountered in these cases.
3.3.3. Imaging Studies
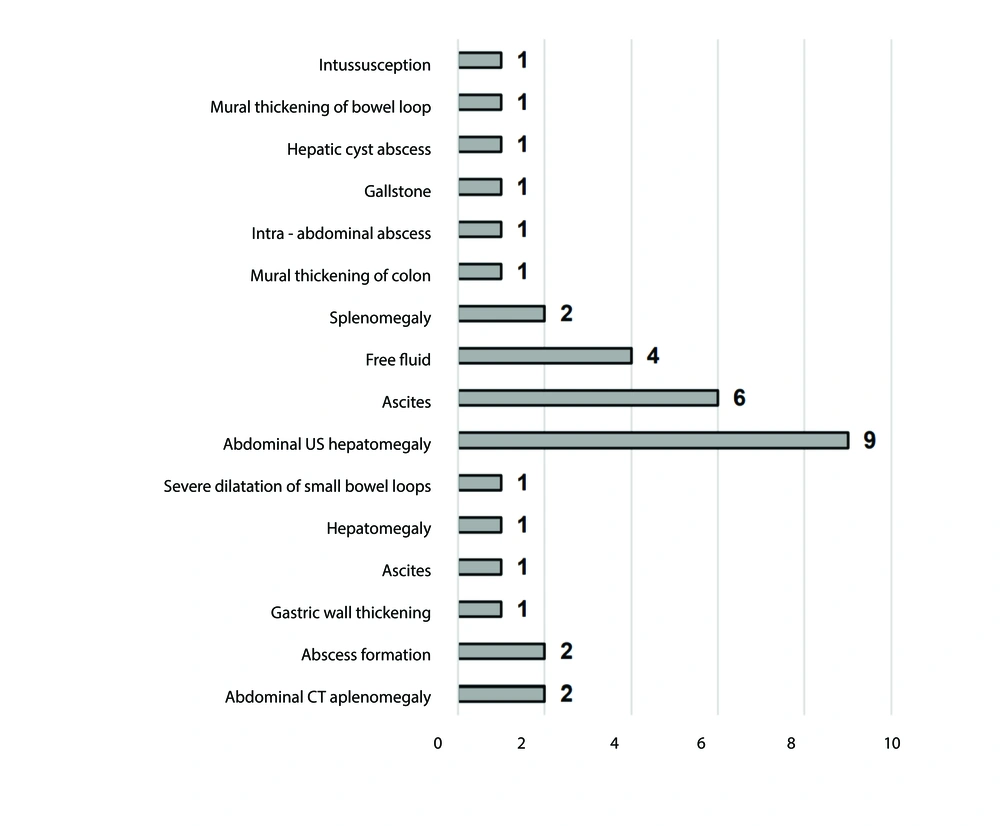
Abdominal computed tomography (CT) or ultrasound (US) was performed to investigate internal organs. Common findings included splenomegaly and hepatomegaly.
3.3.4. Laboratory Tests
Laboratory test results, including blood tests, were collected. Relevant assays included white blood cell (WBC) count, hemoglobin, platelet count, total/direct bilirubin, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and prothrombin time/international normalized ratio (PT/INR).
3.4. Sample Size
The sample size for this study was determined through a census of all available medical records of children diagnosed with immunodeficiency disorders at Mofid Children's Hospital from 2011 to 2022. A total of 43 children were included in the analysis, representing all eligible cases identified during this period.
3.5. Criteria for Performing Endoscopy and Colonoscopy
In this study, the decision to perform upper endoscopy and colonoscopy was guided by the clinical presentation and symptoms, following the guidelines provided in the "Textbook of Gastroenterology", 5th edition by Yamada et al., regarding indications for endoscopic procedures. Indications for upper endoscopy included dysphagia, abdominal pain, or a positive Helicobacter pylori test (8). Indications for colonoscopy included elevated fecal calprotectin, blood in stool, or stool samples containing more than five red or WBCs, along with symptoms such as rectal bleeding, mucus in stool, and chronic diarrhea (9). These procedures were conducted to assess the severity of GI involvement and to obtain histopathological data, guided by the clinical judgment of the attending physicians.
3.6. Statistical Analysis
SPSS software (version 25.0) was used for data description. Continuous variables were described using mean and standard deviation (SD), while categorical variables were described by frequency and percentage. Bar charts were used to represent categorical variables. For percentages and means, 95% confidence intervals (CIs) were reported.
3.7. Ethics Approval
The research adheres to the principles of the Declaration of Helsinki and received ethical clearance from the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran, with the ethical code IR.SBMU.MSP.REC.1397.788 on September 23, 2018. Due to the retrospective nature of the study, the need for informed consent from the participants was waived by the same Ethics Committee.
3.8. Study Bias
The study recognizes potential biases, particularly selection bias from its retrospective design and reliance on a single tertiary care center, which may not reflect the broader population of children with immunodeficiency disorders. To address this, all eligible cases during the study duration were included, strict inclusion and exclusion criteria were applied, standardized data collection forms were used to reduce information bias, and patients with unrelated chronic GI diseases or those receiving immunosuppressive therapy were excluded. Additionally, all medical records were double-checked by two of the authors to minimize bias.
4. Results
Of the 43 children with immunodeficiency evaluated in this study, 25 (58.1%) were male and 18 (41.9%) were female. The mean age was 46.68 ± 51.89 months (3.89 ± 4.32 years). Primary immunodeficiency (PID) was observed in 25 patients (58%), and CID in 18 (42%). The most common immunodeficiency disorder was CID in 11 patients (25.6%), followed by SCID in 6 (14%), interleukin-10 (IL-10) deficiency in 3 (7%), and common variable immunodeficiency (CVID) in 3 (7%). Other immunodeficiency disorders included major histocompatibility complex (MHC) II deficiency, neutropenia, Wiskott-Aldrich syndrome (WAS), autoimmune polyendocrinopathy, Mendelian susceptibility to mycobacterial diseases (MSMD), leukocyte adhesion deficiency type 1 (LAD-1), Shwachman-Diamond syndrome (SDS), congenital neutropenia, early-onset inflammatory bowel disease (IBD), phosphoglucomutase 3 (PGM3) deficiency, and chronic granulomatous disease (CGD).
Table 1 displays the clinical manifestations of the patients. The most frequent clinical manifestations were diarrhea in 24 patients (55.8%) and vomiting in 21 (48.8%). Additionally, the predominant presentation in patients with CID was vomiting (7/11), while patients with SCID most commonly presented with diarrhea (4/6) and poor feeding (4/6) (data not presented in the table). Upper endoscopy and colonoscopy were performed on 14 and 11 patients, respectively (Figure 1). Erythema of the esophagus (3/14), stomach (5/14), and duodenum (3/14) were the most common findings of upper endoscopy. Nodularity of the colon (4/11) and erythema of the rectum (4/11) were the most frequently observed findings in colonoscopy.
| Variables | No. (%) | 95% CI |
|---|---|---|
| Diarrhea | 24 (55.8) | (39.9; 70.9) |
| Vomiting | 21 (48.8) | (33.3; 64.5) |
| Oral/esophageal fungal infection | 15 (34.9) | (21.0; 50.9) |
| Fatigue | 13 (30.2) | (17.2; 46.1) |
| Nausea | 12 (27.9) | (15.3; 43.7) |
| FTT | 12 (27.9) | (15.3; 43.7) |
| Abdominal pain | 9 (20.9) | (10.0; 36.0) |
| Splenomegaly | 9 (20.9) | (10.0; 36.0) |
| Poor feeding | 8 (18.6) | (8.4; 33.4) |
| Hepatomegaly | 6 (13.9) | (5.3; 27.9) |
| Malabsorption | 6 (13.9) | (5.3; 27.9) |
| Anorexia | 4 (9.3) | (2.6; 22.1) |
| Oral aphthous ulcer | 4 (9.3) | (2.6; 22.1) |
| Anal abscess | 4 (9.3) | (2.6; 22.1) |
| Hematochezia | 2 (4.7) | (0.6; 15.8) |
| Jaundice | 2 (4.7) | (0.6; 15.8) |
| Constipation | 2 (4.7) | (0.6; 15.8) |
| Pruritus | 1 (2.3) | (0.01; 12.3) |
| Ascites | 1 (2.3) | (0.01; 12.3) |
Abbreviations: FFT, failure to thrive; CI, confidence interval.
The most frequently observed histopathologic findings were chronic esophagitis (6/14) in the esophagus, chronic gastritis (11/14) in the stomach, chronic inflammation (7/14) in the duodenum, and increased/enlarged lymphoid follicles in the colon (4/11) and rectum (6/11) (Figure 2). Abdominal CT or US revealed splenomegaly and hepatomegaly as the most frequent findings, occurring in 2 out of 43 patients (4.6%) and 9 out of 43 patients (20.9%), respectively (Figure 3). The laboratory test results of the patients are presented in Table 2.
| Variables | Mean ± SD | 95% CI of Mean |
|---|---|---|
| WBC count (/µL) | 10107.57 ± 6621.04 | (8069.92; 12145.23) |
| Hb (g/dL) | 10.39 ± 1.90 | (9.81; 10.97) |
| Platelet count (/µL) | 352297.30 ± 219438.53 | (284764.10; 419830.50) |
| AST (U/L) | 46.40 ± 30.14 | (37.12; 55.68) |
| ALT (U/L) | 38.91 ± 29.12 | (29.95; 47.87) |
| ALP (U/L) | 463.70 ± 189.51 | (405.38; 522.02) |
| Total bilirubin (mg/dL) | 0.57 ± 0.22 | (0.50; 0.64) |
| Direct bilirubin (mg/dL) | 0.22 ± 0.19 | (0.16; 0.28) |
| Albumin (g/dL) | 3.61 ± 0.92 | (3.33; 3.89) |
| PT(s) | 12.60 ± 0.89 | (12.33; 12.87) |
| INR | 1.06 ± 0.14 | (1.02; 1.10) |
Abbreviations: WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; PT, prothrombin time; INR, international normalized ratio; CI, confidence interval.
Table 3 shows that symptoms including nausea, vomiting, abdominal pain, fatigue, and jaundice are more prevalent among patients with CID compared to those with PID. Nausea was reported in 24% of PID children, whereas it was reported in 33.3% of CID patients. Vomiting, the most common symptom, was observed in 61.1% of children with CID, compared to 36% in PID patients. Additionally, physical examination revealed that 24% of PID children had splenomegaly, whereas only 16.7% of CID patients exhibited this condition. The analysis of symptoms specific to PID and CID is presented in Table 3.
| Variables | CID | PID | P-Value |
|---|---|---|---|
| Nausea | 6 (33.3) | 6 (24) | 0.5 |
| Vomiting | 11 (61.1) | 9 (36) | 0.1 |
| Anorexia | - | 4 (16) | - |
| Poor feeding | 2 (11.1) | 6 (24) | 0.28 |
| FTT | 6 (33.3) | 6 (24) | 0.5 |
| Abdominal pain | 5 (27.8) | 3 (12) | 0.19 |
| Jaundice | - | 2 (8) | - |
| Fatigue | 6 (33.3) | 7 (28) | 0.7 |
| Pruritus | 1 (5.6) | - | - |
| Bloody D | - | 3 (12) | - |
| Non-bloody D | 5 (27.8) | 16 (64) | 0.01 |
| Hematochezia | 1 (5.6) | 1 (4) | 0.81 |
| Constipation | 2 (11.1) | - | - |
| Aphthous lesion in mouth | 1 (5.6) | 3 (12) | 0.47 |
| Anal abscess | 2 (11.1) | 2 (8) | 0.72 |
| Hepatomegaly | 2 (11.1) | 4 (16) | 0.64 |
| Splenomegaly | 3 (16.7) | 6 (24) | 0.56 |
| Limb edema | 2 (11.1) | 1 (4) | 0.36 |
| Eyelid edema | 0 | 0 | - |
| Ascites | 1 (5.6) | - | - |
| Liver involvement | 1 (5.6) | 3 (12) | 0.47 |
| Malabsorption | 3 (16.7) | 3 (12) | 0.66 |
| IBD | - | 5 (20) | - |
| GI infections | 1 (5.6) | 3 (12) | 0.47 |
| Fungal infections | 5 (27.8) | 10 (40) | 0.4 |
| Protein-losing enteropathy | 1 (5.6) | - | - |
Abbreviations: FFT, failure to thrive; GI, gastrointestinal; CID, combined immunodeficiency; PID, primary immunodeficiency.
a Values are expressed as No. (%).
5. Discussion
In our evaluation of 43 children with immunodeficiency, most affected by CID, we found diarrhea and vomiting to be the predominant GI manifestations. This is consistent with the research of Akkelle et al., which demonstrated that the most typical presentation in children with PID was persistent diarrhea (1). Moreover, in their study on children with PID, Abdalla et al. reported both diarrhea and vomiting in 39% of patients, without any significant difference between various PID groups (2). While enteropathy may be more specific to PIDs with abnormalities in more than one immune system component, diarrhea is a common symptom across many PIDs (3). This was corroborated by our study, where diarrhea was a frequent finding in SCID patients. Both rotavirus and cytomegalovirus are common GI infections in SCID patients that may lead to malabsorption and recurrent diarrhea (4-6).
Following the respiratory system, the GI tract is the second most affected system by PIDs. The GI system is considered the body’s largest immunological organ and functions as a critical barrier to infections. Infectious, inflammatory, immunological, or malignant conditions may be GI-related in PIDs (7). Along with a variety of commensal bacteria, the gut mucosa and GALT, the body’s largest lymphoid organ, play intricate and crucial roles in the maturation and regulation of the immune system (10). The GI mucosal immune system is directly affected by immunodeficiency disorders. Selective IgA deficiency, CVID, and Bruton agammaglobulinemia are examples of PIDs that can affect B-cell immune response, while DiGeorge syndrome affects T-cell immune response, SCID involves B- and T-cell response, and CGD impacts macrophage and neutrophil defense (11-13).
Three patients in our study had IL-10 deficiency, two of whom experienced diarrhea (data not presented in the table). The IL-10 is a cytokine that regulates inflammation and is secreted by macrophages, regulatory T-cells, and B lymphocytes. Both extra-intestinal and intestinal tissues contain IL-10 and its receptors. Early-onset IBD may be caused by defects in IL-10 and its receptors (14).
Wiskott-Aldrich syndrome was another immunodeficiency disorder identified in this study, affecting the WAS protein, which is essential for B and T lymphocyte signaling. Susceptibility to infection is caused by defects in this protein (15). Colitis, malabsorption, and bloody diarrhea are examples of gut symptoms in WAS (16).
Common variable immunodeficiency was the third most common immunodeficiency disorder in the current study. Numerous inflammatory illnesses of the GI tract, both viral and noninfectious, can adversely affect patients with CVID (17). Due to alterations in the immune system in CVID patients, the definition of GI pathology varies significantly among this population (18). Diarrhea has been reported as a symptom in 20% to 60% of cases (19). GI infections caused by Giardia, Salmonella, Campylobacter, and cytomegalovirus (CMV) are frequently observed in individuals with CVID (20). Noninfectious GI pathologies in patients with CVID include microscopic colitis, celiac disease, lymphocytic gastritis, granulomatous disease, pernicious anemia, acute graft-versus-host disease, IBD, CVID-enteropathy, and small-bowel lymphoma (21). In a study from Italy, GI symptoms in 13 children with CVID included diarrhea, vomiting, dyspepsia, epigastric discomfort, constipation, and abdominal pain (22).
After the lungs, the GI tract is the organ system most often affected in CVID, with up to 50% of CVID patients experiencing GI problems. Moreover, up to 10% of these patients may be affected by non-infectious chronic enteropathy, which might resemble conditions such as celiac disease or IBD (23, 24). Additionally, GI cancers, such as non-Hodgkin B-cell lymphoma, are linked to CVID (25). According to Al-Hussieni et al., CVID patients are more likely to develop infectious diseases in the GI tract, making GI symptoms one of the most significant presentations in these patients. These manifestations might occur early in the course of the disease or later on (26).
The majority of individuals with CVID have recurrent infections due to low antibody levels, characterized by decreased IgG, IgA, and/or IgM levels and limited antibody production (27). In cohorts of the CVID population, reports of IBD mimicking Crohn's disease or ulcerative colitis have been made (25, 28). Reported symptoms include weight loss, persistent diarrhea, rectal bleeding, abdominal pain, and malabsorption. Inflammatory bowel disease -like disease can also manifest as a follow-up to the diagnosis of CVID. Endoscopic characteristics include cobblestone and longitudinal ulcers. Histologically, it can resemble collagenous colitis, lymphocytic colitis, and colitis linked to graft-versus-host disease (19). Tissue pathologic examination indicates a deficiency of plasma cells and an increase in CD81 T-cell infiltrates in the lamina propria. Additionally, compared to controls, lamina propria mononuclear cells from CVID patients may produce higher levels of IL-12 and interferon-gamma, but not IL-23 or IL-17, indicating a different mechanism of inflammation (29).
In the current study, the most frequent findings of upper endoscopy were erythema of the esophagus, stomach, and duodenum. The most typical colonoscopy results were erythema of the rectum and nodularity of the colon. In the study by Akkelle et al., congestion and antral nodularity were the two prominent findings in the stomach, while the esophagus was normal in more than half of the PID patients (1).
Imaging is crucial for diagnosis, determining the degree, detecting complications, and assessing the effectiveness of therapy in GI symptoms of immunodeficiency (30). In patients with suspected GI illness, US is a commonly used, reasonably priced, and safe imaging technique that aids in localizing intestinal abnormalities (31). Important findings suggestive of bowel disease include certain distinguishing characteristics, such as abnormal motility, dilated bowel loops, enlarged mesenteric lymph nodes, interloop fluid, and bowel wall thickening (31). The abdominal US most frequently revealed hepatomegaly, ascites, and free fluid, and less commonly splenomegaly, mural thickening of the colon and bowel loops, intra-abdominal abscess, gallstone, hepatic cyst abscess, and intussusception in our patients.
On the other hand, due to its broad availability and usefulness, CT plays a crucial part in the imaging assessment of GI symptoms of immunodeficiency. A better assessment of intestinal wall thickening, mesenteric lymph nodes, perforation, and extra-intestinal symptoms is possible using CT. It is also used to confirm and evaluate the disease extent and stage of different cancers linked to immunocompromised conditions (32, 33). Consistently, our study revealed splenomegaly, hepatomegaly, abscess formation, ascites, gastric wall thickening, and dilatation of small bowel loops in abdominal CTs of children with immunodeficiency.
In our study, hepatomegaly was observed in 20.9% of the evaluated children, highlighting its relatively high frequency among this population. The presence of hepatomegaly in these children can be attributed to several factors, including chronic infections, inflammation, and the body’s immune response to persistent pathogens (34, 35). Our findings are consistent with previous studies that reported similar frequencies of hepatomegaly in children with PID disorders (36, 37), although some studies reported a higher frequency of hepatomegaly in these children (38).
5.1. Conclusions
The GI manifestations, particularly diarrhea and vomiting, were frequently observed in children with immunodeficiency in our study. Comprehensive evaluations encompassing imaging, endoscopy, and histopathology can provide valuable insights into the extent of GI tract involvement. Larger longitudinal studies are needed to provide a more comprehensive understanding and could validate the findings of this study. Such studies would also allow for the exploration of potential associations between specific immunodeficiency disorders and distinct GI manifestations.
5.2. Limitations and Suggestions
Our study has several limitations. First, the relatively low sample size of 43 patients precludes subgroup analyses regarding GI manifestations and limits the generalizability of the findings. Additionally, abdominal US and CT scans were not performed on all patients, which restricts insights into overall imaging findings. It is also unclear when the GI manifestations developed during the disease course. The retrospective design may have contributed to a lack of accurate and detailed data, and we did not consider the drug history of these patients, even though certain medications can cause GI symptoms. Furthermore, the study's single-center nature in Tehran, Iran, may not reflect the experiences of children with immunodeficiencies in different geographical locations or healthcare systems, and the focus on specific immunodeficiency disorders may not encompass the full spectrum of PIDs. To enhance future research, larger multicenter studies are recommended to validate these results and investigate GI manifestations across diverse settings. Clinically, it is crucial to implement routine GI evaluations for pediatric patients with PIDs, particularly those presenting with common symptoms like diarrhea and vomiting, as early identification of GI complications can significantly improve patient outcomes.