1. Background
Despite notable advancements in perioperative care and surgical practices, the prevalence of cardiac complications after vascular surgery remains a significant clinical challenge (1). Individuals with underlying cardiovascular disease, often characterized by atherosclerosis and associated risk factors like diabetes, hypertension, and dyslipidemia, are more likely to experience adverse cardiac events during and after surgery (2). When these comorbidities are present along with the inherent complexity of vascular surgical procedures, the risk is significantly increased. Cardiac events, especially myocardial infarction (MI), are the leading cause of perioperative mortality (2). Due to their unusual presentations and symptom masking, it can be difficult to diagnose cardiac incidents on time (3).
In primary and secondary preventive contexts, statins — atorvastatin in particular — are beneficial in reducing cardiovascular risk (4). Recent research has revealed a variety of potential pleiotropic effects that statins may have in addition to their well-established lipid-modifying benefits (5). These include enhancing endothelial function, dampening inflammatory responses, and promoting the stabilization of atherosclerotic plaques — actions that may collectively contribute to lowering the incidence of cardiac events post-vascular surgery (5).
However, it is unclear exactly what effect high-dose statin therapy has on perioperative cardiac events, especially in the population undergoing vascular surgery. The benefits of statins for renal protection have been inconsistently reported (6) in the literature, and little is known about how they affect cardiac biomarkers in the context of vascular surgery.
2. Objectives
Against this backdrop, the purpose of our research is to determine whether high-dose atorvastatin therapy can improve cardiac outcomes after lower limb vascular surgery. Our objective is to examine the impact of atorvastatin on a range of cardiac biomarkers, which are prognostic factors that can be associated with cardiac events. Thereby, we seek to shed light on the possible use of atorvastatin as a therapeutic intervention to improve cardiac safety in this group of susceptible patients, thereby influencing perioperative care and enhancing surgical outcomes.
3. Methods
3.1. Study Design
This single-center, double-blind, randomized controlled trial (registered as IRCT20191024045225N1) was conducted at Golestan Hospital, Ahwaz, Iran, between April 2019 and March 2021. The study employed a parallel-group design to evaluate the impact of high-dose atorvastatin on cardiac biomarkers in patients undergoing elective lower limb vascular surgery.
3.2. Sampling Method
1. Consecutive patients scheduled for elective lower limb vascular surgery at Golestan Hospital were assessed for eligibility (n = 94).
2. Fourteen patients were excluded (8 did not meet inclusion criteria; 6 declined participation).
3. Eighty eligible patients were randomized 1:1 via computer-generated allocation concealed in opaque envelopes (40 per group).
4. Intervention: High-dose atorvastatin (80 mg loading dose + 20 mg/day) or control (standard dose).
5. Follow-up: Biomarkers measured at 6, 24, and 72 hours postoperatively.
3.2.1. Inclusion Criteria
Adults ≥ 45 years, ASA physical status II-III, left ventricular ejection fraction > 30%, and ≥ 30 days of preoperative atorvastatin 20 mg/day.
3.2.2. Exclusion Criteria
Recent acute coronary syndrome/cerebrovascular accident, emergency surgery, pregnancy/lactation, or statin hypersensitivity.
3.2.3. Recruitment
Ninety-four patients were screened; 14 were excluded (8 did not meet criteria, 6 declined). Eighty patients were randomized (1:1) into two groups (40 each) using computer-generated allocation concealed in opaque envelopes.
3.3. Intervention Protocols
Participants were randomized (1:1) to: (1) High-dose group: 80 mg atorvastatin loading dose followed by 20 mg daily for 5 days (dosage based on prior clinical evidence) (7); (2) control group: Continued preadmission statin regimen (20 - 40 mg daily).
All participants received standardized perioperative care, including dual antiplatelet therapy (aspirin plus clopidogrel) suspended 7 days preoperatively.
3.4. Outcome Measures
3.4.1. Primary Endpoints
Serial measurements of cardiac biomarkers [troponin I, creatine kinase-MB (CK-MB), creatine phosphokinase (CPK)] at 6, 24, and 72 hours postoperatively.
3.4.2. Secondary Endpoints
Serum creatinine levels and ECG changes (preoperative, 24h, and 72h postoperative).
3.5. Sample Size Calculation
Using established power calculation methods (8), we determined that 40 participants per group (80 total) would provide 80% power (α = 0.05, two-tailed) to detect: (1) Effect size: 0.35 ng/mL in troponin I; (2) assumed SD: 0.5 ng/mL.
These parameters were derived from previous perioperative biomarker studies (9).
3.6. Randomization and Blinding
We implemented (1) computer-generated 1:1 randomization with allocation concealment (opaque envelopes), (2) participants received either high-dose statin or a control group (40 participants each), (3) triple blinding (participants, surgeons, and outcome assessors), and (4) atorvastatin was dispensed in identical capsules.
3.7. Statistical Analysis
Data were analyzed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) with (1) descriptive statistics (mean ± SD for continuous variables; n/% for categorical), (2) independent t-tests for between-group comparisons, and (3) two-way ANOVA for repeated measures (significance threshold: P ≤ 0.05).
4. Results
4.1. Biomarker Analysis
During the study period, 94 patients undergoing lower-limb vascular surgery were initially recruited. Fourteen of them were excluded from the study [not meeting inclusion criteria (n = 8), declined to participate (n = 6)]. Eventually, 80 cases were allocated into two groups of high-dose atorvastatin and control, each with 40 patients. This study was carried out between April 2019 and March 2021 (Figure 1).
4.2. Study Population and Baseline Characteristics
The trial successfully enrolled 80 participants, with balanced demographic and clinical characteristics between groups. No significant differences were observed in (1) mean age (atorvastatin: 54.9 ± 9.9 vs control: 54.7 ± 12.8 years, P = 0.42), (2) gender distribution (male-to-female ratio 16:24 vs 17:23, P = 0.37), (3) comorbidity prevalence: Diabetes (17.5% vs 15%, P = 0.25), hypertension (15% vs 20%, P = 0.29), hypercholesterolemia (17.5% vs 17.5%, P = 0.54), and (4) surgical duration (100.4 ± 25.1 vs 110.1 ± 12.2 minutes, P = 0.76) (Table 1).
| Variables | Atorvastatin Group (N = 40) | Control Group (N = 40) | P-Values |
|---|---|---|---|
| Age (y) | 54.90 ± 9.93 | 54.70 ± 12.78 | 0.42 |
| Sex, n (M:F) | 16:4 | 17:3 | 0.37 |
| Diabetes mellitus | 7 (17.5) | 6 (15) | 0.25 |
| Hypertension | 6 (15) | 8 (20) | 0.29 |
| Hypercholesterolemia | 7 (17.5) | 7 (17.5) | 0.54 |
| Duration of surgery (min) | 100.42 ± 25.14 | 110.11 ± 12.24 | 0.76 |
a Values are expressed as mean ± SD or No. (%) unless indicated.
b Parametric t-test is used for data analysis.
4.3. Biomarker Analysis
All patients showed expected postoperative elevations in cardiac and renal biomarkers. However, the high-dose atorvastatin group demonstrated significantly improved profiles.
4.3.1. Cardiac Markers
Troponin I levels remained consistently lower in the treatment group: (1) 6h: 1.36 ± 0.23 vs 1.68 ± 0.54 ng/mL (P = 0.02), (2) 24h: 1.01 ± 0.41 vs 1.59 ± 0.27 ng/mL (P = 0.02), (3) 72h: 0.77 ± 0.33 vs 1.73 ± 0.29 ng/mL (P = 0.02).
The CK-MB activity showed similar reductions: (1) 24h: 6.62 ± 2.34 vs 8.77 ± 3.73 U/L (P = 0.02) (2) 72h: 4.27 ± 1.11 vs 10.09 ± 3.02 U/L (P = 0.02).
The CPK levels increased comparably in both groups (P > 0.05 at all-time points) (Table 2).
| Biomarker (Within-Group Changes Over Time) | Atorvastatin Group | Control Group |
|---|---|---|
| Troponin I | 6h: 1.36 ± 0.23 → 24h: 1.01 ± 0.41 → 72h: 0.77 ± 0.33 (P = 0.02) | 6h: 1.68 ± 0.54 → 24h: 1.59 ± 0.27 → 72h: 1.73 ± 0.29 (P = 0.12) |
| CK-MB | 6h: 8.56 ± 2.11 → 24h: 6.62 ± 2.34 → 72h: 4.27 ± 1.11 (P = 0.02) | 6h: 9.85 ± 3.45 → 24h: 8.77 ± 3.73 → 72h: 10.09 ± 3.02 (P = 0.15) |
| CPK | 6h: 31.12 ± 4.21 → 24h: 29.78 ± 3.89 → 72h: 30.65 ± 4.02 (P = 0.04) | 6h: 28.07 ± 3.76 → 24h: 22.01 ± 3.12 → 72h: 18.33 ± 2.98 (P = 0.02) |
| Creatinine | 6h: 1.30 ± 0.55 → 24h: 1.17 ± 0.76 → 72h: 1.06 ± 0.42 (P = 0.03) | 6h: 1.41 ± 0.76 → 24h: 1.31 ± 0.34 → 72h: 1.27 ± 0.39 (P = 0.08) |
Abbreviations: CK-MB, creatine kinase-MB; CPK, creatine phosphokinase.
a Values are expressed as mean ± SD.
b Results from two-way repeated measures ANOVA and post-hoc analyses.
| Biomarker (Between-Group Differences at Each Time Point) | 6h Post-Op | 24h Post-Op | 72h Post-Op |
|---|---|---|---|
| Troponin I | 1.36 vs. 1.68 (P = 0.02) | 1.01 vs. 1.59 (P = 0.02) | 0.77 vs. 1.73 (P = 0.02) |
| CK-MB | 8.56 vs. 9.85 (P = 0.07) | 6.62 vs. 8.77 (P = 0.02) | 4.27 vs. 10.09 (P = 0.02) |
| CPK | 31.12 vs. 28.07 (P = 0.03) | 29.78 vs. 22.01 (P = 0.06) | 30.65 vs. 18.33 (P = 0.01) |
| Creatinine | 1.30 vs. 1.41 (P = 0.04) | 1.17 vs. 1.31 (P = 0.04) | 1.06 vs. 1.27 (P = 0.03) |
Abbreviations: CK-MB, creatine kinase-MB; CPK, creatine phosphokinase.
a Values are expressed as mean ± SD.
b Results from two-way repeated measures ANOVA and post-hoc analyses.
4.4. Renal Function
Creatinine levels were significantly lower with atorvastatin: (1) 6h: 1.30 ± 0.55 vs 1.41 ± 0.76 mg/dL (P = 0.04), (2) 24 h: 1.17 ± 0.76 vs 1.31 ± 0.34 mg/dL (P = 0.04) (Table 2 and 3).
4.5. Temporal Patterns
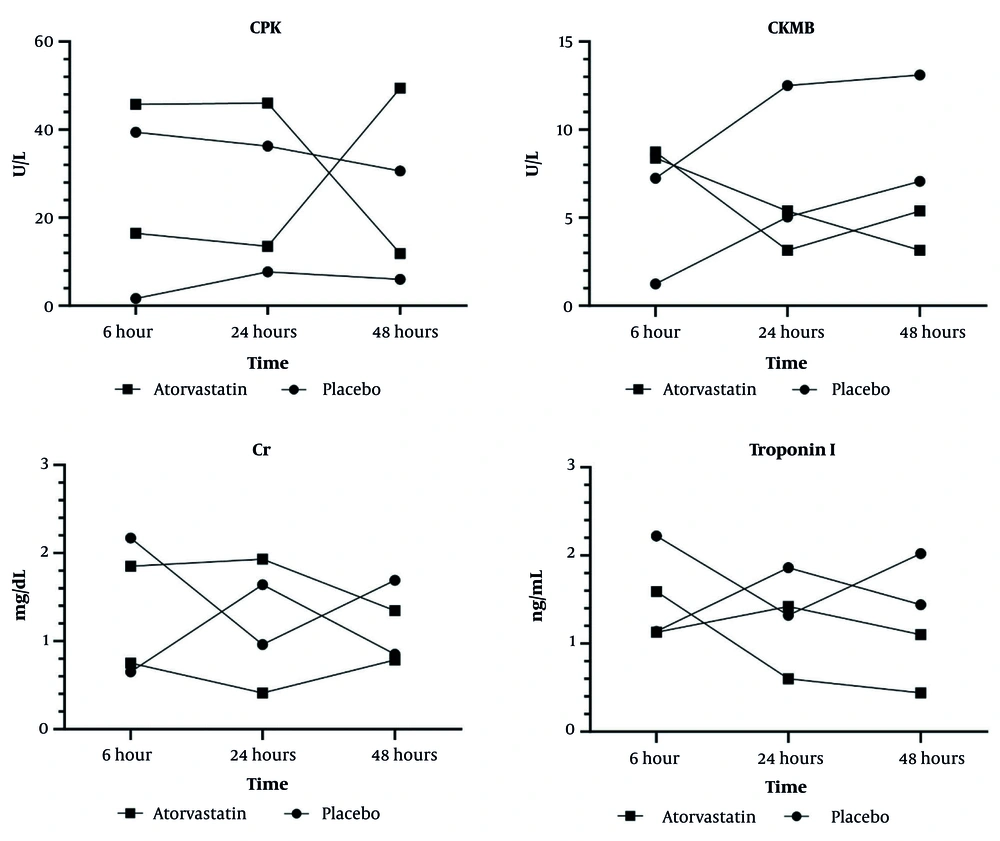
Graphical analysis revealed: (1) Most pronounced biomarker reductions at 6 hours postoperatively; (2) sustained protective effects through 48 hours; (3) consistent advantage across all measured parameters (troponin I, CK-MB, CPK, creatinine) (Figure 2).
Changes in creatinine (Cr), creatine kinase-MB (CK-MB), creatine phosphokinase (CPK), and troponin I levels over time in patients receiving atorvastatin or placebo following lower limb vascular surgery. Values represent mean ± SD. A, significantly different from placebo group (P < 0.05); B: significantly different from atorvastatin at 6h group (P < 0.05); C, significantly different from atorvastatin at 24h group. Two-way measures ANOVA was performed for between-group comparison with “groups” as the between-group factor and “time” as the time × group interaction.
4.6. Electrocardiographic Findings
ECG monitoring showed 24 -72h postoperative changes: (1) Atorvastatin group: Three patients (7.5%), (2) control group: Five patients (12.5%).
No statistically significant intergroup difference (P > 0.05, chi-square test) (Table 4).
| Groups | Atorvastatin | Control | P-Value |
|---|---|---|---|
| Changes after 24 hours | 3 (7.5) | 5 (12.5) | 0.15 |
| Changes after 72 hours | 3 (7.5) | 5 (12.5) | 0.15 |
a Values are expressed as No. (%).
5. Discussion
This randomized controlled trial demonstrated that high-dose atorvastatin significantly reduces postoperative troponin I levels and renal injury biomarkers compared to standard statin therapy in lower-limb vascular surgery patients. The observed mean difference in troponin I at 6 hours (0.32 ng/mL, P = 0.02) suggests a protective effect against perioperative myocardial injury, aligning with growing evidence supporting perioperative statin use in non-cardiac surgery. Our findings show that atorvastatin pretreatment substantially lowered levels of all myocardial injury markers, including troponin I, CPK, and CK-MB. These results corroborate previous studies on statin use in surgical settings, which have consistently reported improved outcomes after non-cardiac procedures (10-12).
Notably, Durazzo et al. found that short-term atorvastatin treatment (≤ 6 months) significantly decreased cardiovascular events post-vascular surgery (8.3% vs. 26.0% with placebo) (13). This consistency strengthens the evidence for statins' cardioprotective perioperative effects. Schouten et al. further confirmed that fluvastatin reduces perioperative myocardial ischemia (RR 0.55, P = 0.01) in vascular surgery patients (14). While their study focused on clinical endpoints, ours extends these findings by quantifying biomarker improvements, highlighting statins' role in mitigating subclinical myocardial injury. In contrast, Liakopoulos et al. reported no troponin reduction with high-dose atorvastatin after CABG (15), possibly due to differences in surgical stress or baseline statin use.
Devereaux et al. established that even minor postoperative troponin elevations (≥ 0.03 ng/mL) predict 30-day mortality (16). Our troponin I reduction (0.32 ng/mL) exceeds this threshold, suggesting clinical significance. Similar effects were observed by another study, where rosuvastatin lowered troponin-T by 0.25 ng/mL in peripheral arterial disease patients (17).
Nicholls et al. demonstrated that high-dose atorvastatin (80 mg) promotes greater plaque regression than moderate doses (40 mg) in stable coronary disease (18). Our study adapts this concept to the perioperative setting, where intensive statin dosing may stabilize vulnerable plaques during surgical stress. However, the STATIN Trial found no renal protection with high-dose atorvastatin post-cardiac surgery (19), underscoring context-dependent statin effects.
The troponin I reduction likely reflects statins' pleiotropic mechanisms:
A. Plaque stabilization: Reduced lipid-core inflammation and matrix metalloproteinase inhibition may decrease plaque rupture risk during hemodynamic changes (20).
B. Ischemia-reperfusion protection: Enhanced eNOS activity improves microvascular perfusion (21).
C. Antithrombotic effects: Lowered platelet aggregation and P-selectin expression may prevent microvascular thrombosis (22).
Our study also noted atorvastatin's influence on postoperative creatinine levels, contributing to the debate about statins' renoprotective role in AKI. While Brunelli et al. reported reduced AKI risk in statin users, particularly after vascular surgery (23), and Molnar et al. found preoperative statins associated with 16% lower AKI incidence and 21% reduced mortality (24). However, other evidence remains inconclusive, as illustrated by Argalious et al., who found no significant association between statin use and improved renal outcomes following non-cardiac surgery (25).
5.1. Potential Harms
While statins are generally well-tolerated, some patients may experience side effects such as myalgia, hepatotoxicity, or, rarely, rhabdomyolysis with high doses of statin therapy. Balancing the potential benefits of perioperative statin therapy against these risks is an important consideration for clinicians and warrants careful patient selection and monitoring. Notably, we observed no significant myopathy or hepatotoxicity despite high-dose therapy, echoing findings by Gao et al. that atorvastatin 80 mg is well-tolerated in acute coronary syndrome (26). The short 30-day perioperative duration may minimize toxicity risks compared to chronic use. The study's small sample size (n = 80) risks under-detecting rare but serious adverse effects of high-dose statins (e.g., rhabdomyolysis) and may overinterpret biomarker improvements without confirming clinical benefit.
In summary, while our results support high-dose atorvastatin's benefits, optimal dosing and duration require further study. Key clinical implications include: (1) Reinforcing statin use for lower-limb vascular surgery patients; (2) suggesting high-dose regimens may offer advantages over standard doses; (3) future research should refine protocols to balance efficacy and safety.
5.2. Conclusions
The study demonstrates that administering high-dose atorvastatin preoperatively significantly lowers cardiovascular and kidney risks in lower limb vascular operations. The observed reduction in biomarker release kinetics suggests a protective effect that extends beyond mere risk reduction, potentially indicating a more comprehensive physiological benefit. Our results demonstrate that, compared to placebo, high-dose atorvastatin administration in the perioperative period significantly attenuates the release of cardiac and renal injury markers.
5.3. Limitations
Our research has several important limitations to consider: Firstly, the small sample size and single-center design may limit the generalizability of the findings. Secondly, the study focused on one type of surgery (lower limb vascular surgery) and may not apply to other surgical procedures. Thirdly, the study did not assess long-term clinical outcomes, such as mortality, morbidity, or readmission rates.
5.4. Clinical Implications
High-dose statins may be a safe, cost-effective plan to reduce perioperative cardiac injury in patients undergoing vascular surgery. To validate these observations and evaluate lasting advantages, future research should involve larger, multi-institutional clinical trials with extended monitoring durations for diverse surgical cases. Further investigation is also warranted to optimize the dosing and duration of statin therapy in the perioperative setting.