1. Background
Breast cancer is the most common cancer among women globally, comprising 25% of all women's cancers. Meanwhile, with an incidence rate of 10%, it is claimed to be the fifth most common cause of death due to cancer in Iranian women (1-4). As with other cancers, hematological abnormalities are common in breast cancer, observed either before or after treatment. However, among all, anemia is the most prominent adverse event, ranging from 30% before treatment to 67 - 80% after chemotherapy, leading to reduced survival and patients’ quality of life, disease progression, and inefficient treatment (5).
Anemia is an important health issue with deleterious consequences on economic and social development as well as patients’ personal lives. Anemia might arise from cancer per se, chemotherapy, bleeding, infection, nutritional deficiency, bone marrow damage, and infiltration by tumor and immunological impairment of the erythropoietic response (6, 7). For instance, chemotherapeutic agents have several negative impacts on bone marrow; firstly, they impede erythropoiesis. Secondly, these agents affect the kidney, preventing erythropoietin production. Finally, these drugs induce anorexia, nausea, vomiting, or diarrhea, resulting in iron or other vitamin deficiencies (8-10). Meanwhile, the effects of chemotherapeutic agents are long-lasting and cumulative; in other words, chemotherapy-induced anemia (CIA) increases from 19.5% after the first cycle to 46.7% after the fifth cycle of chemotherapy (11). Moreover, anemia is caused by a low-grade inflammation existing in cancer patients; in this context, the hemoglobin level would be 8 to 10 g/dL, accompanied by reduced serum iron and transferrin saturation (TS) despite iron stores overload. This phenomenon can be caused by defects in releasing iron rather than iron deficiency, which is known as "functional iron deficiency" (12, 13). As mentioned previously, anemia is a strong predictor of reduced survival and delayed response to chemotherapy in breast cancer patients. In addition, other factors such as the type of cancer, stage and duration of the disease, intensity and type of tumor, concurrent infection, or surgery play a critical role in the prevalence of anemia (10). Treatments for CIA include blood transfusion, which is a rapid and effective treatment in the short term; however, it is associated with some detrimental side effects such as iron overload and increased mortality and morbidity, so they must be used with caution (14). Moreover, erythropoiesis-stimulating agents (ESAs) can be used to treat anemia, even though the risk of thromboembolic events increases and might decline survival and boost the risk of cancer progression or recurrence (15). Other treatment options include oral and intravenous iron therapy to increase the erythropoietic response to ESA and decrease ESA dosage and RBC transfusion. However, in cases of functional iron deficiency, intravenous iron is preferred because the absorption of oral iron supplements from the gastrointestinal tract is trivial (13, 16). According to the European Society of Medical Oncology (ESMO), intravenous iron is indicated for patients with an Hb level less than 11 g/dL and absolute iron deficiency anemia (AIDA; defined as ferritin below 100 ng/dL) or functional iron deficiency anemia (FIDA; with ferritin greater than 100 ng/dL, but TSAT < 20%) before or during the administration of ESAs (17).
2. Objectives
In this study, we aim to investigate the prevalence of anemia, AIDA, and FIDA secondary to chemotherapy in breast cancer patients and their response to intravenous iron after 4 - 6 weeks of therapy over a year.
3. Methods
This study was a cross-sectional study conducted at Motahari Clinic in Shiraz from March 20, 2022, to March 20, 2023. In the study, all non-metastatic breast cancer patients who were assumed to receive adjuvant or neoadjuvant treatment participated, and the prevalence of CIA during chemotherapy courses was evaluated among them. According to the Blood journal, AIDA is defined by having ferritin < 30 ng/mL and TS less than 50%; if ferritin is between 30 - 500 ng/mL and TS < 20%, it is called classic functional iron deficiency. Meanwhile, possible FIDA is characterized by having a ferritin amount of 30 - 500 ng/mL and TS 20 - 50% or ferritin between 501 - 800 ng/mL and TS < 20%. If the ferritin level is above 800 ng/mL and TS is more than 50%, there would not be iron deficiency anemia. These patients were then treated with Ferinject (ferric carboxymaltose), and their response to treatment was assessed 4 - 6 weeks after intravenous iron transfusion. As mentioned previously, the study was cross-sectional, and the sample size took advantage of the Census method, comprising all non-metastatic breast cancer patients who were referred to Motahari Clinic over one year, from March 20, 2022, to March 20, 2023, and met the inclusion criteria. The inclusion criteria were: Patients receiving adjuvant and neoadjuvant treatment and having a hemoglobin level of 12 g/dL or more. On the other hand, the exclusion criteria included: Having anemia (Hb < 12 g/dL) before chemotherapy or having other causes of anemia other than IDA, metastatic disease, chronic renal failure, anemia secondary to previous bone marrow failure, thalassemia, and receiving any blood products or iron supplements during the study without recording.
After the establishment of a final diagnosis of non-metastatic breast cancer, patients who met the inclusion criteria were given informed consent forms. Patients received 4 - 8 cycles of chemotherapy according to their situations, either neoadjuvant or adjuvant chemotherapy, with 2 - 3 week intervals. If patients had a hemoglobin level of 11 g/dL or less or experienced a 1 g/dL hemoglobin drop from their baseline before initiation of each chemotherapy cycle, blood samples such as serum iron, TIBC, ferritin, Coombs (direct and indirect), stool examination, LDH, reticulocyte count, DIC panel, TSH, BUN and Cr, vitamin B12, and folate level were sent. After confirming the diagnosis of "absolute or functional iron deficiency anemia", patients received 500 mg/dL Ferinject in 100 cc normal saline over a 15-minute infusion in two doses, one week apart. Then, after 4 - 6 weeks, the hemoglobin level was measured. It is worth mentioning that patients' privacy was our priority, and it was authorized by the Shiraz Ethics Committee of Medical Science.
3.1. Statistical Analysis
Data were analyzed using IBM SPSS software version 23. Descriptive data were presented as median and frequency; however, the comparison of quantitative data was done using the Mann-Whitney test and paired t-test between two groups, and Kruskal-Wallis and chi-square tests among more than two groups. On the other hand, the Kolmogorov-Smirnov test was utilized to check normality. A P-value less than 0.05 was considered significant.
4. Results
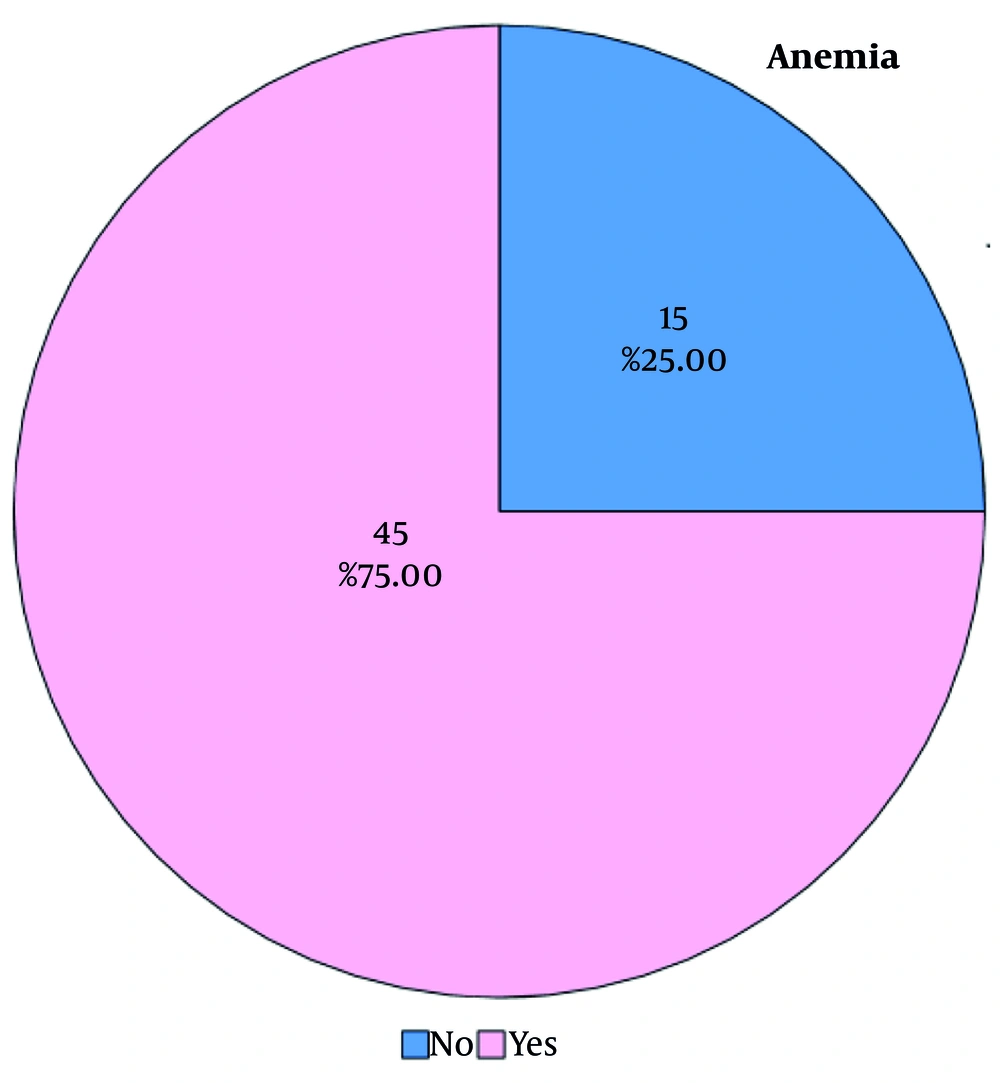
This study was a cross-sectional study in which 91 new cases of breast cancer patients were referred to Motahari Clinic. Out of them, 31 patients were excluded because 12 patients had metastasis, 15 patients had a hemoglobin level less than 12 g/dL before the study, and 4 patients did not want to receive chemotherapy. Sixty patients participated in the study, but 15 patients did not develop post-chemotherapy anemia, and 11 patients experienced post-chemotherapy anemia even though they did not have AIDA or FIDA (Figure 1). Therefore, 45 patients who suffered from post-chemotherapy anemia, FIDA, and AIDA received intravenous iron. The patients’ mean age was 47.4, and the mean hemoglobin level before initiating chemotherapy was 13.3 g/dL.
On the other hand, four patients (6.6%) were stage 1 breast cancer; 18 (30%) and 38 patients (63.3%) were stage 2 and stage 3, respectively. In this study, we did not find any relation between the stage of breast cancer and CIA in the chi-square test (P = 0.891). Regarding the cancer subtypes, the same number of patients were luminal A and B (26 patients altogether), 27 patients had HER2+ breast cancer, and 7 patients were triple-negative. Meanwhile, an association between disease subtypes and CIA was not found (P = 0.971). Out of 60 patients, 22 patients were menopausal, of them 15 patients showed CIA, even though 30 out of 38 pre-menopausal patients experienced CIA. In the chi-square test, we did not find a statistically significant link between menopausal status and CIA (P = 0.372). In addition, the effect of adjuvant and neoadjuvant chemotherapy was evaluated in these patients. Out of 20 patients who received adjuvant chemotherapy, 14 patients presented with CIA; however, among the 40 patients receiving neoadjuvant chemotherapy, 31 patients developed CIA. There was no significant relation between adjuvant and neoadjuvant therapy with CIA in the chi-square test (P = 0.542). The mean age of patients who did not have CIA after chemotherapy was higher compared to those diagnosed with CIA (49.8 and 46.9 years, respectively), and there was no statistically significant association between mean age and CIA in the independent t-test (P = 0.273).
Meanwhile, 52 patients received the Adriamycin (Doxorubicin), Cyclophosphamide (AC), and Docetaxel (T) regimen for 8 cycles (AC for 4 cycles, T for 4 cycles) with 3-week intervals; however, 8 patients received Taxotere (Docetaxel) and Cyclophosphamide (TC) for 6 cycles with 3-week intervals. In addition, HER2+ breast cancer patients also received Trastuzumab. We did not find any relation between types of chemotherapy regimens and CIA in our study (P = 0.22; Table 1). Eventually, we compared the number of chemotherapy cycles. Eight patients received 6 cycles of chemotherapy, while 52 patients received 8 cycles. Patients who received 6 cycles of chemotherapy had a mean hemoglobin level of 13.22 g/dL before initiating chemotherapy and 11.87 g/dL after chemotherapy. On the other hand, the mean hemoglobin level in patients receiving 8 cycles of chemotherapy was 13.3 g/dL before chemotherapy and 11.31 g/dL after chemotherapy. However, in the Mann-Whitney test, there was no significant link between the number of chemotherapy cycles and mean hemoglobin variations (P = 0.226). In contrast, among patients who developed anemia after chemotherapy, we found a remarkable link (P < 0.0001) between the number of chemotherapy cycles and the rate of CIA by applying the MedCalc application. For example, for the first 4 cycles of chemotherapy, patients did not develop anemia, but one patient (2.2%) had anemia after 5 cycles, 3 (6.8%) after 6 cycles, 2 patients (4.4%) after 7 cycles, and 39 (86.6%) showed anemia after 8 cycles.
Finally, out of 45 patients who were diagnosed with CIA, 11 patients did not meet the criteria for FIDA and AIDA, although 34 patients who developed AIDA and FIDA received intravenous iron. Of these patients, 12 (35.3%) had AIDA, 12 (35.3%) developed classic FIDA, and 10 (29.4%) were marked as possible FIDA. Meanwhile, all 34 patients receiving intravenous iron had a mean hemoglobin level of 10.4 ± 0.89 g/dL before treatment, which increased to 11.12 ± 0.73 g/dL after intravenous iron therapy, with a mean hemoglobin variation of 0.714 ± 0.89, which was significant in the independent t-test (P = 0.001) (Table 2). In the Kolmogorov-Smirnov test, three groups of patients were compared; in other words, the mean hemoglobin difference in patients with possible FIDA was 0.22 ± 1.04, with hemoglobin levels of 11.27 ± 0.36 g/dL and 11.49 ± 0.83 g/dL before and after treatment, respectively. Meanwhile, this change for patients with classic FIDA was 0.82 ± 0.65, with hemoglobin levels of 10.25 ± 0.61 g/dL and 11.07 ± 0.68 g/dL pre- and post-treatment, respectively. Finally, patients with AIDA had the mean hemoglobin of 9.83 ± 0.92 g/dL before treatment reaching 10.85 ± 0.63 g/dL after intravenous iron therapy, with the difference of 1.02 ± 0.87
| Classes of CIA (g/dL) | Mean Hb Before IV Iron Therapy | Mean Hb After IV Iron Therapy | Mean Hb Variations After IV Iron Therapy | P-Value Within b |
|---|---|---|---|---|
| Possible FIDA | 11.27 ± 0.36 | 11.49 ± 0.83 | 0.22 ± 1.04 | 0.52 |
| Classic FIDA | 10.25 ± 0.61 | 11.07 ± 0.68 | 0.82 ± 0.65 | 0.001 |
| AIDA | 9.83 ± 0.92 | 10.85 ± 0.63 | 1.02 ± 0.87 | 0.002 |
| Total | 10.40 ± 0.89 | 11.12 ± 0.73 | 0.71 ± 0.89 | < 0.001 |
Determination and Comparison of Hb Level Within Groups Between Time a
At the end, we performed the Kruskal-Wallis test to compare the mean hemoglobin levels in the three groups. The mean hemoglobin level before iron therapy in patients with classic FIDA was remarkably lower than that of possible FIDA (P = 0.001), as was the case for AIDA compared to possible FIDA, with a P-value < 0.0001. However, the association between AIDA and classic FIDA was not significant (P of 0.45).
It should be noted that despite the significant difference in the average changes of hemoglobin in patients with classic FIDA and AIDA compared to possible FIDA, the only statistically significant difference between the effect of iron injection and hemoglobin variation was observed in patients with AIDA compared to possible FIDA in ANOVA and post-hoc tests (P = 0.036). The average changes in hemoglobin after iron injection were not significant between patients with classic FIDA and possible FIDA.
5. Discussion
Despite all advances in the development of new antineoplastic agents, anemia has been troublesome in these patients, with a prevalence of approximately 90%. As outlined previously, anemia can result from cancer itself or cancer treatment, bleeding, and other factors, leading to negative impacts on patients’ quality of life (18, 19). Various treatments can be applied for the treatment of cancer-induced anemia, such as blood transfusion, oral iron supplements, ESAs, and intravenous iron therapy. Even though it has been shown that intravenous iron might not reduce the need for blood transfusion, it is claimed to be more effective than oral iron therapy (20). Meanwhile, intravenous iron therapy has been shown to have a better safety profile compared to oral iron treatment, as IV iron enhances the hematopoietic response and decreases the need for blood transfusion. In addition, the use of ESA is controversial in treating CIA due to some safety problems it presents. Therefore, it has been recommended for palliative care; thus, IV iron might be the safer choice for the treatment of CIA (21). We did not compare the effect of oral and intravenous iron in our study, as based on the mentioned study, we assumed that the effect of oral iron would be trivial compared to intravenous iron. Additionally, gastrointestinal intolerance secondary to chemotherapy and poor compliance would be other negative pitfalls of oral iron. On the other hand, ESA might increase the risk of thrombosis, which is an additional detrimental factor for thromboembolic events in cancer patients (22, 23).
In our study, the prevalence of anemia was 75%, even though our sample size was small. Additionally, we did not conclude that the stage of cancer had a significant relation with CIA. However, in a study performed by Vayrynen et al., anemia is connected with advanced TNM stages. For instance, the prevalence of anemia in patients with stage 0 was 22%, while the prevalence was 30% in stage 1 and 52% in stage 2, and so on, which was not in line with our study (24, 25). This contradiction might arise from the smaller sample size we had. Meanwhile, we did not find a statistically significant link between CIA and the number of chemotherapy cycles in our study; however, the prevalence of CIA increased with the number of chemotherapy cycles. As we mentioned previously, among patients who developed CIA, there was a profound connection between the number of chemotherapy cycles and the rate of CIA. In a study performed by Pourali et al., the prevalence of anemia was higher in patients receiving more cycles of chemotherapy (26).
There was no significant association between different sorts of chemotherapeutic agents and CIA in our study (P = 0.22). However, it has been claimed that anticancer agents might worsen or unmask cancer-induced anemia. Thus, the incidence and severity of CIA depend on the type, dose, and cycles of chemotherapy, and all anticancer therapies might result in anemia, even though the risk is higher in platinum-based regimens (27). Another study performed by Muthanna et al. in breast cancer patients revealed that chemotherapy types had a strong link with the severity of anemia. In the mentioned study, patients who received docetaxel developed a higher rate of anemia compared to those who did not receive this agent, but we did not reach the same conclusion (28).
In our study, out of 34 patients, the same percentage had AIDA and classic FIDA, 35.3%, and 29.4% had possible FIDA. When we compared the mean Hb variations before and after intravenous iron therapy among the three groups, we found that the greatest difference was in AIDA patients, which was 1.02, compared to 0.81 and 0.22 in classic and possible FIDA patients, respectively. However, a study conducted by Abdel-Razeq et al. revealed that the mean hemoglobin was 10.1 g/dL initially, which increased to 12.2 g/dL after 6 weeks, with a mean difference of 2.1. Meanwhile, the response rate was highest among patients with AIDA (80%). The response rate was 70.8% in patients with FIDA, although in this study, FIDA was not categorized into possible and classic FIDA (29).
In another study conducted by Aktas et al. in 2023, the mean change in hemoglobin was 1.8 g/dL after 12 weeks of the first dose of intravenous ferric carboxymaltose treatment. This study had some differences from our study. Even though it showed that the mean hemoglobin level significantly increased after treatment, which was in agreement with our study, it was conducted over a longer period and with a larger sample size of 1,328. In addition, there was no comparison between the effect of treatment on functional iron deficiency and AIDA (30). Meanwhile, another study conducted by Talboom et al. demonstrated the effectiveness of intravenous iron therapy compared to oral iron therapy, with effectiveness rates of 60% compared to 21%, respectively. However, similar to the previous study, there was no comparison or categorization between absolute and functional iron deficiency (31). It seemed to us that intravenous iron therapy might have a profound impact on AIDA rather than FIDA, which has some component of inflammation and suppressing inflammatory factors that reduce the effectiveness of treatment.
On the other hand, despite the increase in average hemoglobin in classic FIDA patients compared to possible FIDA patients, these changes were not statistically significant, which could be due to the small sample size we had.
5.1. Conclusions
In the mentioned study, we concluded that anemia in breast cancer patients responded to intravenous iron therapy, and the most dramatic response was observed in patients with AIDA. Intravenous iron may also be effective in patients with classic FIDA. Further prospective studies with larger sample sizes will be needed to consolidate or refute this claim.