1. Introduction
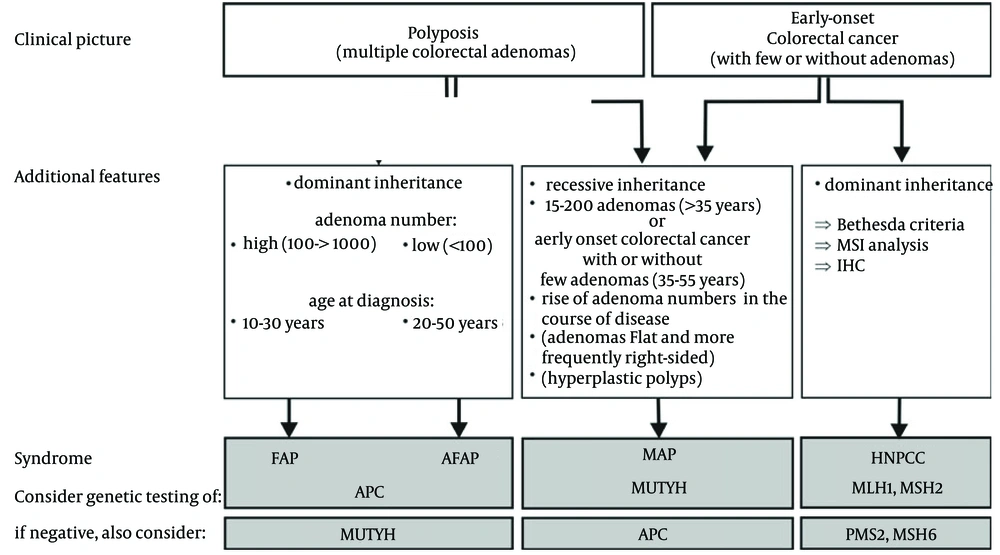
Colorectal cancer develops through a stepwise progression from normal epithelium to adenoma and ultimately to invasive carcinoma. This transformation process is driven by the accumulation of mutations in tumor suppressor genes and oncogenes, which have a background of genomic instability. A number of DNA repair mechanisms function to maintain the integrity of the genome and protect against environmental and endogenous insults (1). Colorectal cancer (CRC) is the second most prevalent cancer worldwide and in 35% of CRC patients, statistically significant effects of hereditary factors have been found (2, 3). The syndromes of CRC are defined on the basis of clinical, pathological, and, more recently, genetic findings. Conditions that express adenomatous polyps include; Lynch syndrome (also called hereditary non-polyposis colorectal cancer), familial adenomatous polyposis (FAP), attenuated FAP, and MUTYH-associated polyposis (MAP) (Table 1). The second best characterized familial syndromes, hereditary non-polyposis colorectal cancer (HNPCC) and familial adenomatous polyposis (FAP), are autosomal dominant inherited disorders and these account for approximately 2% and 0.1%–1% of all cases of CRC respectively (4).
| Syndrome | Associated Gene |
|---|---|
| Adenomatous polyposis syndromes | |
| Familial adenomatous polyposis (FAP) | APC |
| MYH-associated polyposis (MAP) | MYH |
| Non-polyposis syndrome | |
| Hereditary non-polyposis colorectal cancer (HNPCC) | MSH2. MLH1, MSH6, PMS2 |
| Hamartomatous polyp syndrome | |
| Peutz-Jeghers (PJS) | LKB1 |
| Juvenile polyposis (JPS) | SMAD4, BMPR1A |
| Cowden disease, including Bannayan-Ruvalcaba-Riley-syndrome | PTEN |
Inherited Colorectal Cancer Syndromes and Their Associated Genes
Lynch syndrome, or hereditary non-polyposis colorectal carcinoma (HNPCC), is a tumor predisposition syndrome associated with colorectal and endometrial cancer and several other extra-colonic malignancies (5). Although affected individuals can develop colonic adenomas more frequently than the general population, polyposis is rare. Lifetime CRC risk is estimated to be 50% - 80%. Colon cancers and polyps arise in Lynch syndrome at a younger age of onset and at a more proximal location compared to sporadic neoplasms. HNPCC is caused by mutations in the ‘mismatch repair’ (MMR) genes, predominantly MLH1, MSH2, MSH6 and PMS2 (4-6). These tumors show the microsatellite instability (MSI) phenotype. The impairment of the DNA mismatch repair process leads to replication errors and the accumulation of somatic mutations throughout the genome. Somatic slippage mutations occur mainly in DNA repeats, such as microsatellites (6). However, the coding region of tumor-related genes sometimes mutates in mismatch repair-deficient CRC. Somatic mutations in an 8-10 bp mononucleotide repeat are found within the coding regions of the TGF-bRII (7), BAX, TCF4, PTEN and RAD50 genes (8, 9). Mismatch repair systems function as caretakers of the genome (8).
FAP is a well-characterized autosomal dominant disorder in which hundreds or thousands of colorectal adenomas develop, usually during late childhood or early adult life. This inevitably leads to CRC unless prophylactic surgery is performed to remove the large bowel. Although most FAP cases show a pattern of autosomal dominant transmission of the disease, up to 30% of FAP cases are apparently sporadic (10). Patients with FAP may also develop extra-colonic manifestations of the disorder, including skin and bone cysts, duodenal adenomas and cancer, desmoid tumors and asymptomatic retinal abnormalities. FAP is caused by inherited mutations in the APC (adenomatous polyposis coli) gene (11). An attenuated form of the disease, AFAP (attenuated familial adenomatous polyposis), also occurs, and this is associated with smaller numbers of adenomas and later clinical presentation. The risk of CRC is very high, but it typically occurs later in life than in FAP. AFAP is less well characterized than FAP (12). Attenuated FAP is suspected when > 10, but < 100 adenomas, are found in a person older than 40 or 50 years of age. It is caused by mutations in the 3´ and 5´ ends of the APC gene and in the alternatively spliced region of exon 9 (13). Of note, up to 30% of adenomatous polyposis patients do not have germline APC gene mutations (14, 15).
Although their prevalence is low, several other CRC-predisposing syndromes have been described (16). Recent studies have shown that a substantial proportion of patients with multiple adenomas and carcinomas might be associated with a novel type of DNA repair defect. It has been shown that mutations in the base excision repair gene MutY homologue (MYH) might be associated with a new autosomal recessive form of polyposis characterized by the presence of multiple colorectal adenomas and this is associated with a high risk of colorectal cancer (2). MUTYH-associated polyposis (MAP) is an autosomal recessive disorder, which may be responsible for approximately 0.5% - 1% of colorectal carcinomas (CRCs). The majority of biallelic MUTYH mutation carriers are reported to develop multiple polyps (typically between 15 - 200). However, in seven population based CRC studies, proven biallelic MUTYH mutation carriers (38%) had no polyps besides their CRC, while seven (18%) had a limited number of adenomas (17).
2. MutYH Gene and Protein
The MutYH gene, located on chromosome 1p34.3 - p32.1, is 11.2 kb long and contains 16 exons. In addition this gene encodes a protein consisting of 535 amino acids, called MUTYH glycosylase, which was identified in humans in 1995 (1). The MutYH gene is a member of the base excision repair (BER) system involved in oxidative DNA damage repair (18).
Al-Tassan et al. (2002) stated that inherited defects of base excision repair have not been associated with any human genetic disorder, although mutations of the genes mutM and mutY, which function in Escherichia coli base excision repair, lead to increased transversions of G:C to T:A (Nghiem et al., 1988; Thomas et al., 1997). Al-Tassan et al. (2002) studied a British family in which three siblings were affected with multiple colorectal adenomas and carcinoma. There was no clear pathogenic change in the APC gene. They showed that 11 tumors from the three affected siblings contained 18 somatic inactivating mutations of APC, and 15 of these mutations were G:C-T:A transversions. This is a significantly greater proportion than is found in sporadic tumors or in tumors associated with familial adenomatous polyposis. Analysis of the human homolog of mutY, MYH, showed that the siblings were compound heterozygotes for the non-conservative missense variants tyr179-to-cys (Y179C) and gly396-to-asp (G396D) (19). These mutations affect residues that are conserved in the mutY gene of E. coli. Assays of adenine glycosylase activity of the tyr179-to-cys and gly396-to-asp mutant proteins, with 8-oxo G:A and G:A substrates, showed that their activity was reduced significantly. The findings linked the inherited variants in MYH to the pattern of somatic APC mutation in the British family and implicated defective base excision repair in a predisposition to tumors in humans (19).
The MUTYH protein is a base excision repair (BER) glycosylase involved in the repair of DNA damage resulting from the oxidation of guanine nucleotides. Cellular DNA is constantly under attack from damaging agents, such as reactive oxygen species (ROS), which derive from a multitude of exogenous and endogenous sources (reviewed in van Loon et al., 2010). One of the main consequences of ROS impact on DNA is the formation of 8-oxo-G, a frequent DNA lesion estimated to arise around 1 000-7 000 times per cell per day (20). The oxidation product of guanine, 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxoG), readily mispairs with adenosine nucleotides during DNA replication. MUTYH acts by scanning the newly synthesized DNA strand for any mispaired adenines, either with guanines or 8-oxoG's, and excising them.
Two major MUTYH proteins, ie, type 1 and type 2, are expressed in human cells as a result of the presence of multiple transcription initiation sites and the alternative splicing of mRNA transcripts (Takao et al., 1999; Ohtsubo et al., 2000). Type 1 is composed of 535 amino acids, and because it contains a mitochondrial targeting signal (MTS) in its N-terminal, it is localized in the mitochondria. Type 2 is composed of only 521 amino acids, because it lacks the N-terminal 14 amino acids of type 1 which contain the MTS, as a result, type 2 is localized in the nucleus (Takao et al., 1999; Ohtsubo et al., 2000). The excisional repair activity of the type 2 protein is greater than that of the type 1 protein under certain conditions (Shinmura et al., 2000) (21).
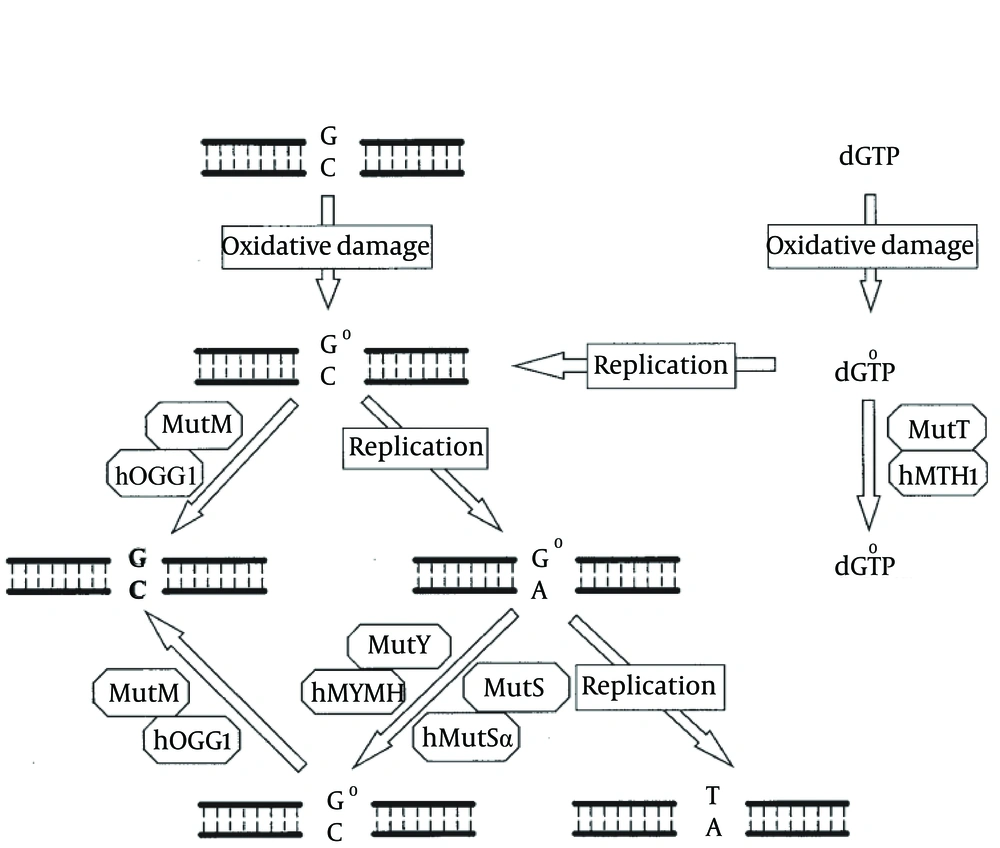
The critical steps in DNA repair by BER in humans are carried out by a set of genes, the human orthologues of MutT, MutM and MutY in bacteria (Tajiri et al.,1995), and these act synergistically to prevent mutagenesis induced by 8-oxoG. These are; nucleoside triphosphatase hMTH1, which hydrolyses 8-oxoG-dGTP, limiting the incorporation of 8-oxo-G in DNA from the nucleotide pool (Sakumi et al., 1993), and the DNA glycosylases hOGG1 and hMYH (the human MutY homolog), which excise 8-oxoG:cytosine (Arai et al., 1997; Boiteux and Radicella, 2000) and 8-oxoG:adenine (A) mismatches respectively, in nascent DNA (Slupska et al., 1999; Shinmura et al., 2000) (Figure 1) (4, 19). On a molecular level, MUTYH has been shown to be physically associated with the MSH2/MSH6 complex via the MSH6 subunit. The MSH6 binding site is mapped to a conserved region in the MUTYH gene and the binding and glycosylase activities of MUTYH are enhanced by the MSH2/MSH6 complex (4).
In E. coli, MutT, MutM, and MutY are involved in defending against the mutagenic effects of 8-oxoG lesions. The MutT protein hydrolyzes 8-oxo-dGTP (dG°TP) to 8-oxo-dGMP (dG°MP) and pyrophosphate. GO (G°) in DNA can be derived from oxidation of guanine or the misincorporation of dG°TP during replication. MutM glycosylase removes GO adducts while it is paired with cytosine. When C/GO is not repaired by MutM, adenines are frequently incorporated in opposite GO bases by DNA polymerase III during DNA replication. A/GO mismatches are repaired to C/GO by the MutY-dependent or MutS-dependent pathway. Cells defective in the MutM, MutY, and MutS repair pathways will have high mutation frequency of G:C to T:A transversions. Human MutY glycosylase homolog (hMYH), 8-oxoG glycosylase (hOGG1), MutT homolog (hMTH1), and MutS homologs (hMutSα), function like their E. coli homologs to protect the cell from the mutagenic effects of 8-oxoG. A dysfunctional MUTYH protein increases the occurrence of somatic G > T transversions. For instance, somatic mutations in the APC gene in MAP tumors involve almost exclusively G > T transversions, an observation that led to the discovery of the MAP syndrome. Similarly, the most prevalent KRAS2 mutation in MAP tumors is a G > T transversion at codon 12 (c.34G > T), which was reported to be present in 64% of MAP carcinomas (17).
3. Identification and Characterization of MAP (MYH-Associated Polyposis)
Clinical diagnostic criteria for MAP have not yet been fully established, although some of the features of the studies conducted to date can be estimated. MAP patients can present with conventional adenomas as well as serrated adenomas, hyperplastic polyps and mixed (hyperplastic and adenomatous) polyps. In 47% of MAP patients one or more hyperplastic polyp and/or sessile serrated adenoma (SSAs) were found (4). Additionally, G:C to T:A transversions in KRAS were identified in 51 of 73 (70%) hyperplastic or sessile serrated polyps in individuals with MAP, compared with only 7 of 41 (17%) in their sporadic counterparts (22). This evidence indicates an association between MAP and hyperplastic or sessile serrated polyps (5). The majority of the adenomas in biallelic mutation carriers were tubular or tubule - villous histological types (18).
MYH-associated polyposis often has an attenuated phenotype in terms of the age of onset and numbers of adenomas compared with classic familial adenomatous polyposis (23). Typically, an MYH-associated polyposis patient has a cumulative adenoma count of 15-200 (22-24), while adenoma counts of 1,000 have never been reported (13, 25). Most studies have reported frequencies between 10% - 30%, with the highest rate in AFAP patients with homozygous or compound heterozygous and germline MYH mutations.
In total, 60% of MAP patients have CRC at the time of diagnosis (26). Some MAP patients have only CRC and no polyps (27). Most MAP patients have around 100 adenomas at diagnosis, with a mean age of about 45 years, and they develop CRC at a mean age of about 50 years (27). In eight population-based CRC studies, proven biallelic MUTYH mutation carriers (35%) had no polyps aside from their CRC, while seventeen (22%) had a limited number of adenomas. Age at diagnosis of CRC, when compared to sporadic cases, was relatively young in MAP patients (47 and 49 years). MAP CRCs showed less metastases than sporadic CRCs, but more than Lynch carcinomas (17). MUTYH screening should be directed at patients with between 10 and a few hundred polyps (adenomas and/or hyperplastic polyps) and patients with CRC at 30-50 years, especially in the context of a family history that is compatible with recessive inheritance, although a vertical transmission of CRC does not rule out the possibility of biallelic MUTYH mutations (4).
4. Mutation spectrum
Non-truncating mutations appear to cluster in known functional domains of MutYH, while truncating mutations have been identified throughout the coding region, and the majority of MUTYH mutations were missense mutations, in addition, only a few represent splice site, frameshift, or nonsense mutations (28). The c.536A > G (Y179C) and c.1187G > A (G396D) mutations have been found in more than 80% of Caucasian patients with MAP in 7 and 13 exons (29-31). Other possibly recurrent pathogenic MYH mutations have been reported in specific ethnic groups, that is, Y90X, E466X, 1395delGGA, and 1187insGG, in subjects of Pakistani, Indian, Italian, and Portuguese descent, respectively (1).
5. MutYH Mutation and CRC Risk
Although large population-based studies are lacking, it is estimated that about 1 in 2 500 to 10 000 individuals have biallelic MUTYH mutations, and the lifetime risk of colorectal cancer is estimated to be 80% (14). It has become widely accepted that homozygous and compound heterozygous germline MYH mutations predispose to the development of colorectal adenomas and carcinoma in an autosomal recessive manner (1).
CRC was found in 65% of biallelic MYH mutation carriers, and the mean age of onset was 47 years. The majority of studies found no evidence that monoallelic mutations increase CRC risk. There has been much debate regarding whether monoallelic (heterozygous) mutation carriers also have a higher risk for developing colorectal cancer, possibly at a later age (4). If such ‘second hits’ are associated with the acquisition of a mutator phenotype at the cellular level, then predisposition to CRC might result, however, a definitive estimate of this risk has not been determined with certainty. Peterlongo et al. hypothesized that monoallelic MUTYH mutations are enriched in MMR-gene mutation-negative HNPCC-like CRC families in which the MUTYH mutation constitutes a low penetrance CRC-causing allele. In another study, monoallelic MUTYH mutation carriers had a positive family history in 58% (7 of 8 cases) for monoallelic MUTYH mutation carriers with vertical transmissions, suggesting an elevated risk of CRC in relatives with a similar dominant inheritance of CRC cases, this allows the hypothesis of a disease-causing synergism of MUTYH mutations with other genes (18). Racha Khalaf et al. postulated that a single mutation is sufficient to increase the risk of colorectal cancer. They also proposed that the G382D MYH mutation may play a dominant rather than a recessive role in polyposis and cancer development (32). For heterozygous relatives of MAP patients, the available data suggest that such individuals have a two or at most a three-fold increase in their risk of CRC at an age similar to that of the general population, and thus are expected to benefit from population screening measures or they could be offered average moderate-risk colorectal screening based on their family history (22).
6. Molecular PROFILE of MAP Tumors
The reason why mutations cause the development of adenoma and colorectal carcinoma is not clearly understood. However, several studies have shown that changes such as G > T are common in the APC gene in tumor DNA from MAP patients (increase of about 20% - 80%). The majority of these changes result in the formation of a stop codon and thus a truncated protein product. For this reason, similar to mismatch repair genes in HNPCC, the MYH gene is thought to be a ‘caretaker’ gene, where MYH inactivation increases the mutation rate, compared with the ‘gatekeeper’ APC gene where mutation initiates neoplasia directly (33). Whether these changes are present at the initiation of adenoma formation is uncertain. In the APC gene, mutations tend to target TGAA or AGAA motifs. Thus, creating stop codons, and resultantly a truncated APC protein. Theoretically, of course, mutations G > T can occur at any location of the APC gene (4, 34). Moreover, it has been shown that K-ras (a poto-oncogene) is frequently mutated in MAP adenomas and cancers. Surprisingly, all K-ras mutations contain the same G12C (G > T) change. In further studies a specific KRAS mutation (the c.34 G > T in codon 12) was found in 64% of CRCs (4). In one cohort of 192 cases, 10 tumors had a somatic c.34G > T KRAS2 mutation (6 carcinomas and 4 adenomas) (35). MAP cancers do not appear to display microsatellite instability or chromosomal instability and they show a near-diploid karyotype with low overall levels of loss of heterozygosity (13).
7. Genotype - Phenotype Correlation
In E. coli it has been found that the corresponding mutation of Y179C has a more deleterious effect on the rate of adenine removal than the corresponding mutation of G382D. In addition, heterozygous Y179C mutation carriers are associated with a higher risk for CRC than G382D heterozygotes. A recent study has shown that the phenotype for MAP patients with biallelic p.G396D mutations was less severe than for Y179C homozygotes. Patients with a homozygous p.G396D mutation or compound heterozygous p.G396D/p.Y179C mutations presented with MAP later and they had a significantly lower risk of developing CRC than patients with a homozygous p.Y179C mutation. The mean ages of CRC diagnosis were 58 years (homozygous p.G396D), 52 years (c. heterozygous p.G396D/p.Y179C) and 46 years (homozygous Y179C) (4). In the largest genotype-phenotype study of MAP patients, researchers found that Y179C homozygotes presented earlier and they had a significantly greater CRC possibility than G396D homozygotes and G396D/Y179C compound heterozygotes. Furthermore, they also found that compound heterozygosity for G396D and a second mutation, other than G396D or Y179C, was associated with a more severe phenotype (ie, higher CRC hazard and earlier presentation) than G396D homozygosity or G396D/Y179C compound heterozygosity (26).
8. MYH and Sporadic Cancers
There is evidence to show that somatic MYH mutations occur very rarely (if at all) in sporadic colorectal tumors, and loss of expression is also probably very rare (34). Most genes that result in a very high risk of CRC when mutated in the germline, have also been shown to be subject to somatic inactivation in sporadic CRC. APC is mutated in the germline in FAP and it is subject to biallelic somatic mutation in the majority of colorectal adenomas and carcinomas (30). The MMR genes are mutated in the germline in hereditary non-polyposis CRC, and MLH1 is inactivated (usually via promoter methylation) in some 10% - 15% of sporadic CRCs. Germline mutations of SMAD4 are associated with juvenile polyposis (hamartomatous colorectal polyps and later CRC risk), while somatic inactivation is associated with the later stages of adenoma progression (31). In one study, Halford et al. found no somatic mutations of MutYH in any of the 75 unselected CRCs or 35 CRC cell lines. MutYH mRNA and protein were expressed in all the cell lines, indicating that epigenetic silencing was also unlikely to occur at a significant frequency (13), and in addition, another study only found one mutation present in two patients in a heterozygote state of 48 sporadic CRCs (18).
9. Extracolonic Manifestations in MAP
Since oxidative stress is a common manifestation, it can be expected that a defective MUTYH gene will lead to cancers and tumors external to the colon as well. Indeed, in MUTYH knockout mice, tumors have been found in the small intestine (36). Mice deficient in both MUTYH and APC (APC min/+) have tumors in the small intestine, breast and lung. In double MUTYH and OGG1 knockout mice, ovarian tumors and lymphomas were found. Several studies have reported extra colonic lesions, mostly in the upper gastrointestinal tract, in MAP patients. Duodenal lesions (37), gastric lesions (22), and breast cancer (20), were all relatively common in MAP.
In another study it was shown that biallelic cases had a high incidence of extracolonic polyposis in 32% of the cases. In total, 32% had polyposis of the small intestine or gastric polyps, and one case had polyposis of the gall bladder. None of the whole patients were reported with manifestations, such as congenital hypertrophy of the retinal pigment epithelium (CHRPE), desmoids or osteomas (18). A European study showed that gastric and duodenal polyps occurred in approximately 11% and 17% of patients, respectively, with an estimated lifetime risk of duodenal cancer of 4%. FAP-associated extracolonic features such as; osteomas, desmoids, congenital hypertrophy of the retinal pigment epithelium, and thyroid cancer, did not occur, but an excess of ovarian, bladder, skin, sebaceous gland tumors, and possibly breast cancer, was observed (12). In addition to colorectal polyposis and cancer, adenomatous polyps of the duodenum and gastric fundic gland polyps are common in MYH-associated polyposis and duodenal cancers have been reported (33).
10. Discussion
Germline MYH mutations predispose to recessive inheritance of multiple colorectal adenomas, colorectal cancer, and classic adenomatous polyposis in a variety of populations (38). All patients with biallelic MYH mutations probably have an increased risk of colorectal cancer. In patients with a phenotype of classic polyposis and no detectable APC mutations, about 10%-30% of cases result from germline MYH mutations. In addition, a number of MAP patients have been described with CRC, with none or only a few polyps. It may be difficult to distinguish between patients with germline APC and biallelic MYH mutations on the basis of clinical and pathologic features. Family history can be useful in this context, although a number of MAP patients will have a parent affected with colorectal cancer by chance. Genetic testing for MYH is warranted for any isolated patient with 15-200 colorectal adenomas (or 5 adenomas if presenting younger than 35 years), or colorectal carcinoma and having a family history, although we would test APC first if more than a 1 000 adenomas were present (Figure 2).
MYH mutation screening should initially be focused by ethnic origin, and on the mutations known to be common in those populations, even if the whole gene is eventually screened in those without two of the common mutations. Germline MYH genetic testing should be offered to first-degree relatives of carriers and given that the greatest risks are associated with biallelic inheritance of mutations, carrier spouses should be offered genetic testing to afford the best counseling possible for at-risk offspring (33).