1. Background
The prevalence of obesity is increasing. Obesity contributes to some chronic diseases such as cancer and increases the risk of several types of tumors, which may have high costs of treatment. Cancer is a global epidemic and prevalence of total cancer is increasing in developed and developing countries. One-third of total cancers are due to dietary and life style patterns and obesity is one of the main risk factors for many cancers. According to previous studies, obese subjects had higher risk for developing cancer than normal weight subjects (1). There are some strategies for treating obesity. Although diet therapy and physical activity are the best methods for weight control and obesity treatment (2, 3), however, in some conditions weight loss medications are necessary (4). Today, many drugs are used to decrease body weight and decrease adverse outcomes of obesity such as cancer. Orlistat is an anti-obesity drug that is approved by the Food and Drug Administration and is used for treating obesity. Orlistat promotes weight loss, improves lipid profiles and blood pressure and compared with other medications has greater efficiency for weight maintenance after weight loss. Mechanisms associated with the anti-obesity effects of orlistat are inhibition of pancreatic and gastric lipases and inhibition of dietary fat absorption (5). According to recent studies, orlistat has beneficial effects on progression of cancers and exhibits anti-proliferative and antitumor properties. Orlistat induces apoptosis and delays tumor growth in several cancer cells. Orlistat is a novel inhibitor of some enzymes that are strongly linked to tumor progression, however its mechanisms have not been understood fully (5, 6).
Different mechanisms have been suggested regarding the antitumor activity of orlistat yet findings in this regard have been controversial. Orlistat is an available medication and has less negative side effects than other medications for treatment of obesity. Once the mechanism of action of this medicine on tumor proliferation has been identified, this drug can be used as an antitumor drug in addition to an anti-obesity drug in the near future.
2. Objectives
The aim of this review was to describe the effect of orlistat as an antitumor agent on growth of cancer cells.
3. Patients and Methods
Articles were identified from data-bases such as Google Scholar, PubMed, Scopus and Proquest. Medical subject heading (MeSH) key words used to search the database included orlistat and obesity, orlistat and neoplasm, orlistat and proliferation, antitumor and orlistat, and fatty acid synthase and orlistat. Forty-nine articles were found. We excluded articles that were not in the English language or published prior to 2005. Finally 25 articles were selected that were published from 2005 until the present and investigated orlistat function on cancer progression. Designs of articles were experimental, clinical trial and case reports.
4. Results
4.1. Inhibition of Fatty Acid Synthase (FASN) by Orlistat
Fatty acid synthase (FASN) is an enzyme for synthesis of long chain fatty acids from acetyle coenzyme A (CoA) and malonyl CoA. In normal human cells, expression of FASN is low (except liver and adipose cells). Over-expression of FASN as a neoplastic marker is needed for synthesis of cellular membrane of tumor cells in proliferation, and is positively associated with progression of many human cancers. Fatty acid synthase is an attractive target for cancer therapy (6). Many studies have suggested that ceasing of FASN activity can decrease growth of tumor cells and can be considered as an anti-cancer therapy (7).
Fatty acid synthase has seven catalytic domains, including thioesterase. According to previous studies, orlistat inhibited the thioesterase domain of FASN. Thus, inhibition of FASN is one of the mechanisms of action of orlistat that influence cell proliferation. Studies have shown that orlistat reduces growth of prostate cancer cells in vitro and in vivo, breast tumor cells, gastric tumor cells, melanoma and ocular cancer cells by inhibition of FASN and enhancement of apoptosis (8). However, in another study orlistat had a modest anticancer effect on prostate cancer and a high concentration of orlistat stimulated breast cancer cells in vivo (9).
Orlistat with inhibition of FASN can block tumor cells proliferation, stimulate tumor cell apoptosis and decrease viability (5). According to cell cycles analysis, orlistat, with a dose dependent manner, decreases G2-M phase and S-phase, and increases sub-G1 (apoptotic) cells. In tumor cells, FASN activity increases phospholipid biosynthesis for membrane cells. Phospholipids biosynthesis increases during G1 and S phase and orlistat can arrest the cells at the G1-S phase.
Tumor cells induce angiogenesis for survival. Studies have shown that orlistat, by inhibition of FASN activity cells, reduces metastases and tumor-induced angiogenesis. In another study orlistat inhibited FASN of endothelial cells and proliferation of cells; also inhibited neovascularization in an ex vivo investigation. So orlistat can act as an anti-angiogenic drug (10).
4.2. Effect of Orlistat on Colon Cancer
Study on colorectal cancer showed that orlistat significantly inhibited cells proliferation and increased level of caspase-3 and apoptosis compared with the control group in vitro. Antitumor effect of orlistat was dose dependent. Furthermore, 25 mM of orlistat inhibited 50% of cells proliferation and 50 mM increased this inhibition. In addition, an in vivo study showed that orlistat inhibited cells proliferation. However, activation of FASN didn't change unless in very high doses such as 200 mM of orlistat (6).
In contrast to these findings, some studies suggested that orlistat might increase risk of colon cancer. Garcia et al. (11) showed that orlistat at various concentrations had indirect genotoxic effects. Aberrant crypt foci (ACF) significantly increased in orlistat treated rats and orlistat enhanced cell proliferation. Aberrant crypt foci and hyper-proliferation are risk factors of colon cancer progression and they are premalignant lesions. Orlistat accumulated undigested fat in the colon and produced free radicals that stimulated cell proliferation in colonic epithelium. Production of reactive oxygen species (ROS) in feces leads to lipid per-oxidation. These oxidative agents damage lumen membranes and enhance cell death. In addition, lipids per-oxidation leads to production of cytotoxins.
4.3. Effect of Orlistat on Lymphoma Tumor Cells
The findings of one study showed that orlistat, in a dose dependent manner, significantly reduced the number of mantle cell lymphomas (MCL), increased apoptosis and decreased cells viability. In addition, orlistat increased caspase activity (12). Kant et al. (13) found that orlistat could change the survival of lymphoma tumor cells and inhibit proliferation of tumor cells. In this study 75 µM of orlistat at 48 hours of treatment significantly inhibited FASN activity compared with the untreated group. They reported that orlistat significantly increased intracellular ROS in T cell lymphoma, which was associated with FASN inhibition and induced apoptosis. Agostini et al. (14) also found that orlistat inhibited FASN and significantly reduced proliferation and promoted apoptosis. In this study, mice that were treated with orlistat had lower tumor volume and proliferation index compared with the control group. They showed that the orlistat treatment group had 43% less metastatic lymph nodes than the control group.
4.4. Effect of Orlistat on Breast Cancer
The effect of orlistat has also been investigated on breast cancer. Expression of FASN and Her2/neu (an oncogene that is over-expressed approximately in 30% of breast carcinomas) usually increases in breast cancer. Findings of a previous study showed that Her2/neu over-expression induced the promoter of FASN gene and thus increased lipogenesis (5). Orlistat induced an anti-proliferative effect on breast cancer cells through suppression of Her2/neu expression and blockage of the activity of FASN. Menendez et al. (9) showed that orlistat induced poly ADP-ribos polymerase (PARP) and finally affected apoptosis by caspase on breast tumor cells. Similar mechanisms were observed in ovarian cancer. Researchers found that orlistat inhibited FASN, suppressed Her2/neu oncogene expression and induced cytotoxic effects on ovarian cancer cells in vitro (15). Huang et al. (16) investigated the effect of orlistat on protein expression involved in antitumor activity. They found that orlistat significantly decreased protein expression involved in tumorigenesis of human ovarian cancer and down-regulated these proteins.
4.5. Effect of Orlistat on Hydrolysis of Anticancer Drugs
Orlistat can affect other drugs that are used for cancer treatment and influence their efficacy. Xiao et al. (17) suggested that orlistat can inhibit the hydrolysis of anticancer drugs, including carboxylesterase (CESs) hydrolyze drugs, xenobiotic and lipids. Three human CES were recognized as CES1, CES2 and CES3. The CES2 hydrolyzes many common drugs and is a major enzyme in metabolism of drugs, preferably hydrolysis of anticancer agents. Orlistat inhibits CES2 and consequently decreases hydrolysis of anticancer drugs and prolongs their efficacies. Increase concentration of orlistat leads to enhancement of interdiction properties. The CES2 was sensitive to orlistat and 1 nM of orlistat inhibited 75% of CES2 activity. Thus, anticancer drugs and orlistat can be co-recommended as a new therapeutic plan. Some studies have suggested that orlistat acts on tumor cells through affecting tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Furthermore, TRAIL is one of the targets for cancer therapy. Some tumor cells are resistant to TRAIL and according to previous findings, orlistat enhances the sensitivity of prostate tumor cells to TRAIL. Also, orlistat stimulates expression of death receptor 5 (DR 5), which is the TRAIL receptor. Orlistat induced both mRNA transcription and translation of DR5 (18).
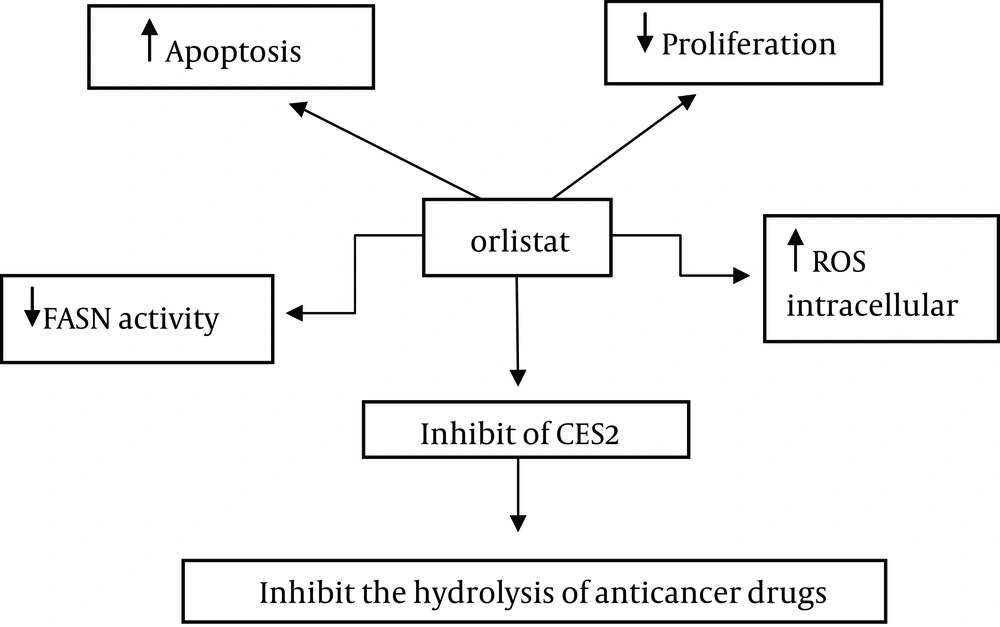
4.6. Mechanism of Anti-Cancer Effects of Orlistat
Summary of mechanisms of cancer cells growth inhibition by orlistat was shown in Figure 1. Previous findings have suggested that orlistat has toxic effects on tumor cells and inhibits growth of cancer cells and enhances apoptosis without affecting normal cells. The selective cytotoxicity of orlistat was seen in colon cancer (5). There aren't any long term studies to explain the function of orlistat on proliferation of cells and its possible genotoxic and cytotoxicity effects (19). Controversial findings about the effects of orlistat on tumor cells make this subject difficult to discuss. In contrast to recent studies that have reported the anti-tumor action of orlistat, Orsolin et al. (19) didn't find any anti-carcinogenic effects and tumor reduction as a result of orlistat intake. Calderon et al. (20) reported that orlistat decreased glutathione and serotonin and finally decreased the activity of the oxidative stress defense system. In addition, orlistat disturbed absorption of fat-soluble vitamins such as vitamin A and vitamin E and these vitamins are important in antioxidant defense and prevent oxidative damage of DNA. These changes lead to weakness of the immune system, damage cells and stimulate tumor cells production and mutagenic effects. The difference in the results of various studies may by due to different concentrations of orlistat and study designs and populations.
There is a hypothesis that an increase in fecal fat can affect colonocytes via the cytotoxic effect of free fatty acid and secondary bile acids that are risk factors of colon cancer (21). It seems that orlistat accumulates undigested fat in the colon and is associated with colon cancer. Ahnen et al. (22) didn't find any evidence to support the hypothesis that colonocyte proliferation rate increases with orlistat. They studied healthy obese men and women and found that orlistat significantly increased fecal weight, total fecal fat and fecal free fatty acids, while secondary bile acids decreased in the orlistat treatment group and orlistat didn't change colonic cell proliferation.
Although some studies suggested orlistat as an anti-cancer medication, yet oral orlistat has low bioavailability, low metabolic stability and low solubility (23). Due to these limitations, novel formulation of orlistat will be required for tumor cells treatment. Oral formulation of orlistat could be useful for treating tumors of gastrointestinal tract (GI) because oral orlistat can directly contact tumor cells of GI and affect these cells. Thus, orlistat as an anticancer drug would likely be restricted to GI tumor treatment since approximately 1% of orlistat is absorbed (9).
Orlistat may affect some tumor serum markers and lead to misdiagnosis. Findings of the first case report in this regard, showed that carcinoembriogenic antigen (CEA) elevated during orlistat usage. Carcinoembriogenic antigen is a serum marker that elevates in 80% of patients with tumors. It is a clinical tool for follow-up of patients with several types of tumors. This report showed that when orlistat usage was stopped, CEA level returned to its normal ranges. Thus orlistat may cause false positive CEA elevation and these findings should be considered in management and treatment of patients (24). The summary of studies that investigated the effect of orlistat on cancer cells are shown in Table 1.
| Reference | Design of Study | Orlistat Dosage | Results of Study |
|---|---|---|---|
| Chuang et al. 2011 (6) | In vivo treatment (mice) | 0-200 µM of orlistat for 72 hours | Orlistat caused cell cycle arrest at G1 phase, increased apoptosis through caspase-3 activation. Tumor size of orlistat-treated mice in vivo was significantly smaller than controls with 55% inhibition. FASN is a potential target for the treatment of human colorectal carcinoma. |
| Menendez et al. 2005 (8) | In vitro treatment (gastrointestinal carcinoma cell) | 10 μM of orlistat for 48 hours of treatment | Orlistat blocked FASN activity and gastrointestinal (GI) carcinoma cell proliferation and blocked GI cell cycle progression. Orlistat decreased the expression of Her-2/neu oncogene by more than 90%. |
| Menendez et al. 2005 (9) | In vitro treatment (human breast cancer cell line) | 0 to 20 µM of orlistat for 72 hours | Orlistat can be considered as a novel therapeutic drug for treating Her2/neu over-expressing breast carcinomas and inhibiting FASN activity. |
| Garcia et al. 2006 (11) | In vivo treatment (Male Wistar rats) | 200 mg/kg of chow for 30 days | Orlistat significantly increased the number of colonic aberrant crypt foci (ACF) and cell proliferation |
| Gelebart et al. 2012 (12) | In vitro treatment (mantle cell lymphoma cell line) | 0 to 20 µM of orlistat for 48 hours | The expression of FASN was detectable and high in mantle cell lymphoma (MCL) and was negative in normal cells. The data supports the concept that FASN contributes to the pathogenesis of MCL and FASN inhibitors such as orlistat may improve treating MCL. |
| Kant et al. 2012 (13) | In vitro treatment (murine model of a T cell lymphoma) | 75 μM of orlistat for 48 hours | Orlistat inhibits FANS activity, enhances apoptosis and increases intracellular ROS production in tumor cells. |
| Fernandes et al. 2010 (24) | Case report (66-year-old female patient) | 120 mg of orlistat, three times per day for weight control for 6 months | Orlistat can be the cause of false positive elevation of carcinoembriogenic antigen (CEA) (elevated CEA level of 8.3 ng/ dL (baseline 3.0 ng/dL)) |
| Xiao et al. 2012 (17) | In vitro treatment (pooled liver microsomes from humans, mice or rats) | Various concentrations (0-1000 nM) of orlistat were tested for hydrolytic activity | Orlistat significantly inhibited hydrolysis in human and mice microsomes. CES2 was profoundly reduced upon incubation with orlistat in human and mice microsomes. |
| Orsolin et al. 2012 (19) | In vitro treatment (somatic cells of Drosophila melanogaster) | Three different concentrations of orlistat (2.4, 4.8, and 9.6 mg/mL) | Orlistat does not have carcinogenic potentials and cannot reduce tumors induced by mitomycin C in D. melanogaster |
| Ahnen et al. 2007 (22) | Twenty-four obese (body mass index, 30-40 kg/m2) but otherwise healthy male and female subjects | 120 mg of orlistat, 3 times a day for 6 weeks | Treatment with orlistat significantly increased fecal weight, total fecal fat, and fecal free fatty acids compared to the placebo. Orlistat did not alter colonic cell proliferation |
a Abbreviations: FASN, fatty acid synthase; GI, gastrointestinal; ACF, aberrant crypt foci; MCL, mantle cell lymphoma; ROS, reactive oxygen species; CEA, carcinoembriogenic antigen; CES, carboxylesterase.
5. Discussion
Orlistat is an approved anti-obesity drug and in comparison with other anti-obesity drugs has fewer side effects. According to the anti-cancer effect and inhibition of FASN by orlistat, by changing the oral formulation, a novel drug with more bioavailability and absorbency power can be produced for the treatment of cancers. In addition, with this drug we can treat obesity that is a risk factor of cancers. Due to different findings regarding the efficacy of orlistat on cancer prevention or co-recommendation with other anti-cancer drugs, it seems that recommendation of orlistat for high-risk colon cancer patients is not favorable. However, the design of many studies that investigated the effect of orlistat on cancers was in vitro and these findings cannot be generalized to humans. Thus we need more in vivo studies to understand the effect of orlistat on human cancer cells. In conclusion, more research is needed to understand the effect of orlistat on fat accumulation in the colon and to find the other mechanisms of action of orlistat on cells and finally to ensure the safety of orlistat for human treatment plans.