1. Background
Attention deficit hyperactivity disorder (ADHD) is a disease that is not well understood even though genes associated with it have been identified. ADHD is one of the most common psychiatric problems in childhood and adolescence (1, 2). An estimated 5% to 12% of school-age children suffer from ADHD (3). The disease can negatively affect the social interactions of children and adolescents. Although the disease mainly affects boys, it can also affect girls (4). It has been suggested that the noradrenergic system consisting of a central system (locus coeruleus) and the peripheral sympathetic system is involved in the etiology of ADHD (5-7). The peripheral sympathetic system plays a greater role in the incidence of ADHD symptoms, as a decrease in the peripheral’s epinephrine levels lowers the ability of the locus coeruleus to function (6).
In many studies the levels of epinephrine and norepinephrine or their metabolites have increased in the plasma and urine of a case group of children with ADHD compared to those of a control group without ADHD (5-8). However, there are no consistent findings among these results that indicate an increase, a decrease, or no change in the peripheral level of monoamines or their metabolites. Regarding the role of the central noradrenergic system and the automatic peripheral sympathetic system in regulating the arousal state and due to the noradrenergic effects of stimulant drugs, the hypothesis was developed that the noradrenergic system is involved in ADHD (9-12).
Another physiological disorder associated with ADHD is the reduced response of the adrenaline moderator (5).This reduced response can also be seen during cognitive tests and even during the development of hypoglycemia. It indicates a condition called epinephrine slowed response (13). It is hypothesized that this slowed response is caused by the increase of peripheral epinephrine described above. Among the most common treatments of this disorder are stimulant medications such as methylphenidate (5-7, 14). By facilitating the release of catecholamines in the locus coeruleus and controlling their resorption in the cell synapses, stimulant medications increase the levels of these substances and thus decrease the symptoms (5, 8, 14).
ADHD has three subtypes: predominantly impulsive-hyperactive, predominantly inattentive, and combined. Both pharmacological and non-pharmacological treatments of this disorder have been proven effective. Methylphenidate is the most common drug that has been proven affective for all three types of ADHD symptoms (15-17). The response to this drug in children and adolescents varies from 25% to 78% (18).
Stimulant medications are the first-line pharmacological treatment for ADHD. Their side effects, including tics, irritability, and loss of appetite, have made it necessary to find alternative treatments with fewer side effects or complementary medications in order to alleviate the remaining symptoms (6).
The role of zinc has been proven in the neurotransmission of immune system activities, growth and development, some hormonal activities, the function of the taste and smell senses, and the healing of injuries. The essential role of zinc in protein and nucleic acid syntheses has also been proven (19-21). Magnesium plays a fundamental role in the energy-production conversion of ATP to ADP (22). Suboptimal levels of nutrients such as zinc, calcium, and magnesium have been reported for some children with ADHD. There are also contradictory results among previous research done on the positive effects of ADHD treatments. For these reasons, this paper seeks to investigate the therapeutic effect of the combination of zinc, calcium, and magnesium as a supplementary treatment in a population of children with ADHD in the city of Zahedan.
2. Methods
2.1. Study Design
This clinical trial was conducted on 40 children aged between 6 to 12 years who were referred to public and private clinics in the city of Zahedan in 2014.
2.2. Participants
Children aged 6 to 12 years with a definite diagnosis of ADHD participated in the study. After selecting patients and receiving permission from the children’s parents, the method and purpose of the study were explained to them. The exclusion criteria included the presence of any major concurrent psychiatric illnesses other than oppositional defiant disorder and learning disability, an IQ lower than 70, use of psychotropic drugs or narcotics within the past two weeks, the presence of any important neurologic diseases, and taking any drugs affecting the nervous system at least two weeks prior to the study. A demographic data questionnaire was then completed for each child separately. Before beginning the treatment, a physical examination was done on the participants, including their weight, blood pressure, and heart rate. The ADHD Rating Scale diagnostic questionnaire was also completed for the participating children. The parental version of this scale was completed by one parent of each child as well. The minimum score of the questionnaire necessary for study participation was 20, and the response to treatment was based on a reduction of at least 25% in symptoms compared to the baseline of the ADHD rating scale.
2.3. Treatment Protocol
The patients were randomly divided into two groups using a random numbers table. The case group was treated with methylphenidate twice a day. The dosage started at 0.3 mg/kg and was increased to 1 mg/kg within two weeks. The case group was simultaneously treated with tablets containing 133 mg zinc, 333 mg calcium, and 5 mg of magnesium. Patients in the control group received the same dose of methylphenidate and a placebo. In the weeks 2, 4, 6, and 8 of treatment, the ADHD Rating Scale questionnaire was completed again for both groups by a psychiatrist’s assistant. The information regarding drug side effects was collected based on the interviews with the patients and their parents and the observations of the therapist according to the drug side effects questionnaire.
2.4. Randomization and Double-Blind Protocol
The medication prescriber and the patient investigator for the study were separate people. During the study, the medication prescriber and the patient investigator were not aware of the type of prescribed medications. To make it a double-blind study, the researcher randomly divided the participants into the case and control groups. Each patient was given a code and referred to the person in charge of drug distribution. Methylphenidate and the zinc, calcium, and magnesium supplement were given to the patients based on the codes 1 and 2 on the packets, which were prepared in advance.
2.5. Statistical Analysis
The data analysis was done using the statistical software SPSS, Version 18.0. Numerical variables were presented as a mean (SD), while nominal and categorized variables were summarized by absolute frequencies and percentages. For comparing the baseline measurements between the two groups (age, sex, dose of methylphenidate, and number of responders), a chi-square test, t-test, and paired test were used.
2.6. Research Ethics
The proposal for this thesis research was presented to the ethics committee of Zahedan University of Medical Sciences after its scientific approval by the Child and adolescent psychiatry department. This study was also registered in the Iranian registry of clinical trials (irct.ir) with the ID: IRCT2015092624209N1.
3. Results
The mean age of the children in the case group was 9.6 ± 1.5 years and that of the children in the control group was 8.9 ± 1.6 years (P = 0.235). Of the children, 32 were boys (80%) and 8 were girls (20%) (P = 0.429). Figure 1 shows the CONSORT flow diagram of the trial.
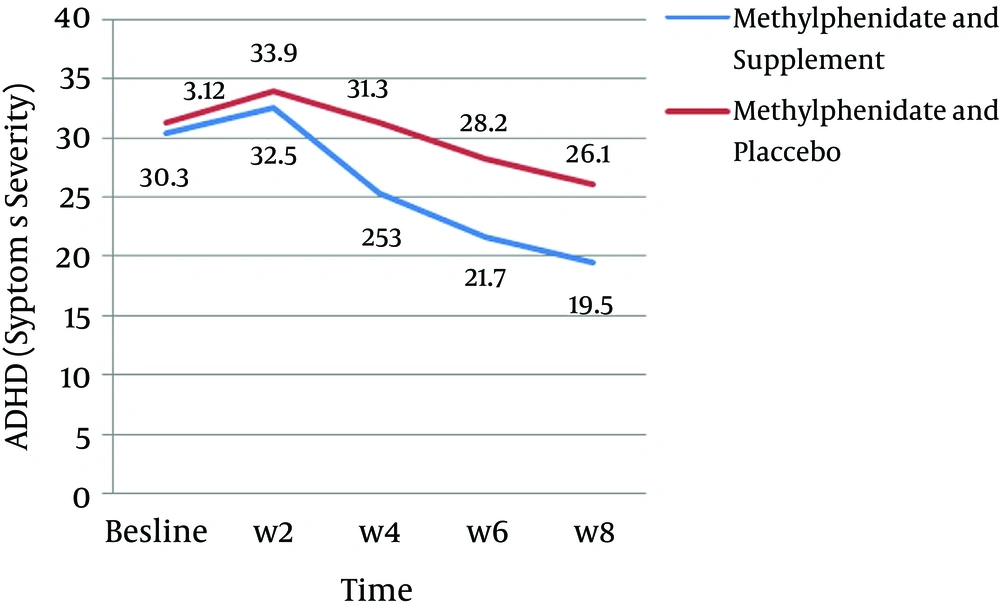
In the present study, the means for symptom severity in the case group before and 8 weeks after the treatment were as follows: for the predominantly inattentive type (before: 20.5 ± 2.5 and after: 8.9 ± 3.1), for the predominantly impulsive–hyperactive type (before: 19.9 ± 2.8 and after: 10.6 ± 4.1), and for the combined type (before: 40.4 ± 2.4 and after: 19.5 ± 6.1). In addition, the means for symptom severity in the control group were as follows: for the predominantly inattentive type (before: 20.7 ± 2.8 and after: 12.9 ± 3.7), for the predominantly impulsive–hyperactive type (before: 20.5 ± 2.9 and after: 13.2 ± 5.9), and for the combined type (before: 41.2 ± 2.7 and after: 26.1 ± 9.0) (P < 0.001) (Table 1, Figure 2).
| Group/Type of Symptoms | Pre-Intervention | Post-Intervention | P Value |
|---|---|---|---|
| Case | |||
| Predominantly inattentive | 20.5 ± 2.5 | 8.9 ± 3.1 | < 0.001 |
| Predominantly impulsive-hyperactive | 19.9 ± 2.8 | 10.6 ± 6.1 | < 0.001 |
| Combined | 40.4 ± 2.4 | 19.5 ± 6.1 | < 0.001 |
| Control | |||
| Predominantly inattentive | 20.7 ± 2.8 | 12.9 ± 3.7 | < 0.001 |
| Predominantly impulsive-hyperactive | 20.5 ± 2.9 | 13.2 ± 5.9 | < 0.001 |
| Combined | 41.2 ± 2.7 | 26.1 ± 9 | < 0.001 |
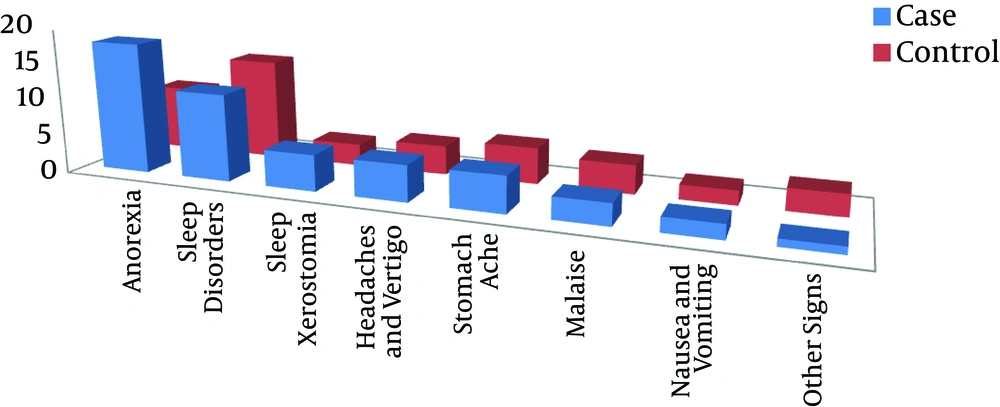
The mean for symptom severity after treatment in the case group showed a significant decrease for the predominantly inattentive and combined types in comparison to the control group (P < 0.05) (Table 2). The main side effects of methylphenidate for the two groups were anorexia and sleep disorders, and anorexia was significantly greater in the case group (P = 0.002). Furthermore, the side effects of the supplements included dry mouth, nausea, and abdominal pain in two of the children (Figure 3).
| Type of Symptoms | Case | Control | P Value |
|---|---|---|---|
| Predominantly inattentive | 8.9 ± 3.1 | 12.9 ± 3.7 | 0.001 |
| Predominantly impulsive–hyperactive | 10.6 ± 6.1 | 13.2 ± 5.9 | 0.114 |
| Combined | 19.5 ± 6.1 | 26.1 ± 9 | 0.01 |
4. Discussion
The results of the present study indicate that the mean for symptom severity in the case group was significantly reduced after 8 weeks of treatment. Moreover, it was noticeably reduced in the control group after the same time period. The results also show that the reduction of symptoms in the case group was greater than that of the control group.
Several similar studies have been conducted and it has been shown that micronutrients such as magnesium and zinc are associated with the treatment of ADHD symptoms (23-28). Most of these studies have focused on zinc and are consistent with the findings of our study. For example, a study by Arnold et al. was conducted on 48 children and suggested that lower levels of zinc were associated with inattentiveness in children with ADHD (29). In another study in 2011, Arnold et al. stated that taking zinc supplements alone would reduce ADHD symptoms in children (30).
In addition, Akhondzadeh et al. studied the use of zinc supplements in combination with pharmacological treatment in 44 children and better improvement in the symptoms was seen compared to taking medications alone (31). Oner reported that ferritin and zinc deficiencies were associated with symptoms of hyperactivity in children (32). These results are consistent with our study. However, the double-blind trial by Zamora et al. showed different results and suggested that taking a daily 10 mg zinc supplement had no effect on reducing ADHD symptoms (33).
Zinc indirectly affects dopamine metabolism and increases methylphenidate tendency for dopamine transmission. Scientific evidence suggests that dopamine is a critical factor in ADHD. Magnesium is also essential for physiological and biochemical processes and can control the N-methyl-aspartate glutamate channel that plays a role in apoptosis. Magnesium likewise affects brain catecholamines. Therefore, the lack of these micronutrients can increase the severity of ADHD symptoms (28). A study by Nogovitsina and Levitina indicated a reduction of plasma and erythrocyte magnesium levels as well as the reduction of Mg (2+) ATPase activity in children with ADHD (19).
Approximately 15% of the zinc in the brain is found in synaptic vesicles, from which it is released to the extra neuronal space during synaptic transmission. Around the synapse, zinc acts upon a variety of neuronal receptors and ionic channels, playing a modulator role that is not yet fully understood (34) .Within the vesicles, zinc is a cofactor for the production of dopamine from l-dope, serotonin from 5HTP, and melatonin from serotonin. Hence, zinc is likely to be an important modulator of synaptic transmission (35). Research suggests that zinc may regulate methyl-d-aspartame receptors (35) and animal studies have shown that zinc is an important regulator of gamma-amino butyric acid receptors, affects the excitability of hippocampus glutamatergic neurons, and may play an important role in cerebellar function (36). Recent studies have shown evidence for a significant reduction of the combined glutamate/glutamine to creatine ratio in the right anterior cingulate cortex in patients with ADHD (37) and striate glutamate, glutamate/glutamine, and creatine concentrations were higher in ADHD subjects than in control subjects, providing evidence of a striatal creatine/glutamatergic deregulation in ADHD patients.
Magnesium acts mainly by: a) the reduction of presynaptic glutamate release; b) the reduction of NMDA receptor activity by competing with calcium at NMDA receptor coupled calcium channels; c) the positive allosteric modulator effect at the level of some metabotropic presynaptic glutamate receptors, thereby decreasing presynaptic glutamate release and stimulating GABA release; and d) the decrease of catecholamine release by a direct presynaptic effect under the action of some factors including calcium (38).
Furthermore, Mahmoud et al. concluded that levels of zinc, iron, and magnesium in children with ADHD are lower than in healthy children (24). This is in agreement with a previous study by Nogovitsina and Kozielec that showed magnesium levels were significantly lower in children with ADHD than in the control group (20, 26).
In a 14-month study conducted by Vazir et al. on a population of over 608, it was suggested that micronutrient supplements improved the symptoms associated with attention and concentration in the participating students (11). Schoenthaler et al. studied the effect of dietary supplements on aggressive and antisocial behaviors in children, adolescents, and adults. They found evident improvements in patients with a proven nutritional deficiency in their red blood cells. The most common nutritional deficiencies included pyridoxine, folic acid, thiamin, niacin, vitamin C, iron, calcium, copper, magnesium, zinc, selenium, manganese, and molybdenum (13, 14).
4.1. Conclusions
This study shows that a zinc, calcium, and magnesium supplement is effective in the treatment of ADHD.