1. Background
Various hypotheses have been proposed with respect to neurochemical, biochemical, genetic, and immunological abnormalities associated with the onset and progress of schizophrenia. During the past decade, the roles of the immune system and cytokines in the pathophysiology of schizophrenia have received much attention (1-4). According to the literature, schizophrenic patients have abnormal levels of immune-competent cells, cytokines, and cytokine receptors, especially proinflammatory interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-12p40, and the IL-2 receptor, in their peripheral blood or cerebrospinal fluid (5-8). There is also evidence for the existence of antibrain antibodies in the serum of schizophrenic patients (9). Cytokines, which act as chemical messengers between immune cells, modify the metabolism of neurotransmitters and neuroendocrine hormones and influence neural development and behavioral changes, as supported by basic scientific studies (10, 11).
In this study, we examined the levels of two major cytokines: interleukin-23 (IL-23) and IL-6. The finding that stimulation of activated and/ or memory T cells in the presence of IL-23 but not IL-12 induced the production of IL-17 but not IL-4 or IFN-γ provided the first evidence for a unique role for IL-23 in the modulation of Th1 cell-responses and the regulation of T-cell effecter function (12-14). Further studies showed that macrophages and microglia within the CNS were targets of IL-23 and that this interaction stimulated local production of TNF-α by these cells. IL-23 was shown to induce a subset of T cells, with a unique cytokine expression pattern (IL-6, IL-17A, IL-17F, and TNF-α), sufficient to induce neurological disease (15). Consistent with this finding, blockade of IL-17A alone but not IFN-γ decreased the severity of clinical disease in this model. Another study reported that IL-23 increased the proliferation of CD4+ memory T cells and the production of IFN-γ that conveyed to a pattern in which naive T cells are not responsive to IL-23 but activated or memory Th1 cells were sensitive to the effects of this cytokine (16).
IL-6 is expressed in the CNS from activated astrocytes and microglia cells. Several studies have suggested that IL-6 might be involved in deterioration during autoimmune disorders in the CNS (15) and that cytokines, such as IL-6, TNF-α, and IL-2, were important mediators of interactions between the immune system and the CNS (17, 18).
In schizophrenic patients, various patterns of cytokine release and psychotic behavior have been proposed (7, 19). Therefore, it is reasonable to suggest that the presumed immune pathology in psychiatric disorders might lead to changes in circulating cytokine levels. Studies using IL-23-deficient mouse models clearly demonstrated that IL-23 was an important element of CNS autoimmune inflammation (20).
2. Objectives
In this study, serum IL-23 and IL-6 concentrations of drug-free schizophrenic patients and healthy controls were investigated.
3. Patients and Methods
3.1. Subjects
In this case-control study, we recruited 30 schizophrenic patients (20 males, 10 females) from Baharan psychiatric hospital in Zahedan in the southeast of Iran. The hospital is a governmental, specialized referral center for psychiatric patients. The control group consisted of 20 students and medical staff (14 males, 6 females) who had not been diagnosed with any psychiatric disorders and had no medical or familial history of psychiatric diseases. The average age of the patient group was 34.3 ± 7.1 and that of the controls was 32.2 ± 6.9. This research was approved by the ethics committee of Zahedan University of Medical Sciences (code: 90-193; date: 29 April, 2012).
The diagnosis of schizophrenia in all cases was based on a structured clinical interview and the DSM-I criteria of the American psychiatric association (1994) for schizophrenia. All the patients were experiencing their first episode of schizophrenia symptoms and had not received any psychotic drugs for at least 2 months before the study. The median duration of the disease was 3.05 ± 2.6 years. A complete medical history was obtained from all the patients, and they all underwent a physical examination and laboratory tests. Subjects with ongoing infections, allergies, a past history of autoimmune disorders, or other medical morbidities that might cause a change in inflammatory status were excluded. According to the aims of the study, the controls and patients were matched in terms of gender and age. All the subjects gave written informed consent, and the study was approved by the institutional review board of Zahedan University of Medical Sciences. Each patient’s antipsychotic medication remained the same for 6 weeks: clozapine (100 - 350 mg/day, n = 16), risperidone (3 - 5 mg/day, n = 8), olanzapine (20 mg/day, n = 6), and risperidone (37.5 - 50 mg/2 weeks, n = 0).
3.2. Blood Sampling and Measurement of Serum Levels of IL-23 and IL-6
Venous blood (8 mL) was collected between 9: 00 am and 12:00 p.m. and was allowed to clot at room temperature. The serum was obtained by centrifugation at 2000 × g for 10 minutes as early as possible and kept frozen at -80 until assayed. The IL-23 and IL-6 levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) (eBioscience) for quantitative detection of human IL-23 and IL-6. The sensitivity of the assay was 4 pg/mL for IL-23 and 0.92 pg/mL for IL-6. All the assays were carried out concurrently. Hematological and biochemical examination findings were within the normal range in all the patients, and all the patients were free of acute infections and allergic reactions.
The results are presented as the mean ± SD (standard deviation), and a P value of less than 0.05 was considered statistically significant. Data on the age and body mass index (kg/m2) and duration of the disease were recorded. Statistical analysis was performed using SPSS 17 (SPSS Inc., Chicago, IL, USA). Serum IL levels showed a non-Gaussian distribution and were analyzed by appropriate nonparametric tests. The Kruskal-Wallis test was applied for a comparison between independent groups, and the Mann-Whitney test was used for a comparison between dependent groups.
4. Results
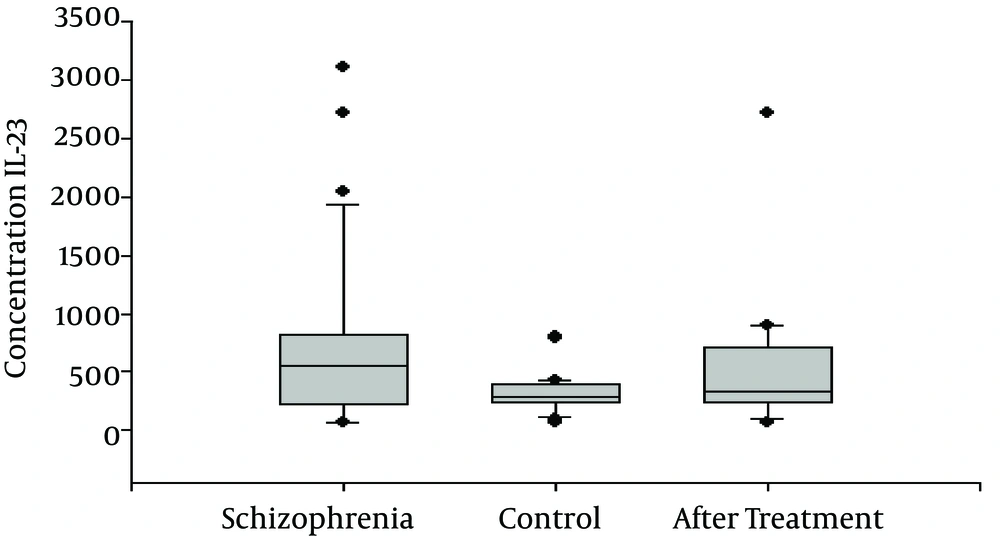
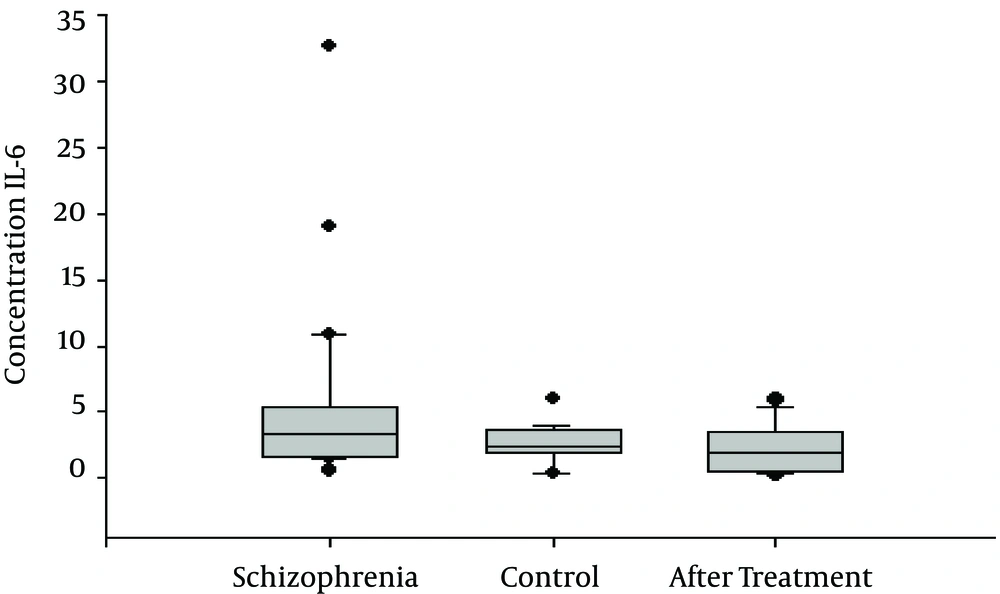
The serum levels of IL-23 and IL-6 of the 30 schizophrenic patients and 30 age- and gender-matched healthy controls were measured using an ELISA assay. All the samples were assayed in duplicate, and the same plate was used. IL-23 and IL-6 were detectable in the serum samples of all the subjects. As shown in Figure 1, the serum IL-23 levels of the schizophrenic patients were significantly higher than those of the control subjects ((696 ± 132 pg/mL and 313 ± 33 pg/mL, respectively, P = 0.007). The serum IL-6 levels were also significantly increased in the patients with schizophrenia (5.28 ± 1.1 pg/mL) when compared to those of the control group (2.54 ±0.32 (pg/mL; P = 0.01), as shown in Table 1 and Figure 1. After 6 weeks of medical treatment, there were no significant differences in the IL-23 levels of the schizophrenic patients versus those of the controls (696 ± 132 pg/mL and 511.52 ± 105 pg/mL, respectively; P = 0.3). However, IL-6 appeared to be significant after 6 weeks of medical treatment compared with the group before treatment (5.28 ± 1.1 pg/mL vs. 2.35 ± 0.36 pg/mL), as shown in Table 2 and Figure 2.
| Factor | Schizophrenia Group | Control Group | P Value |
|---|---|---|---|
| IL-23 | 696 ± 132 | 313 ± 33 | 0.007 |
| IL-6 | 5.28 ± 1.1 | 2.54 ± 0.32 | 0.01 |
| Age | 35.1 ± 10.68 | 32 ± 6.1 | NS |
| BMI (kg/m2) | 22.2 ± 3.9 | 21.7 ± 2.7 | NS |
Abbreviations: BMI, body mass index.
aValues are expressed as Mean ± SD.
| Factor | Schizophrenia | Schizophrenia Six Weeks After Treatment | P Value |
|---|---|---|---|
| IL-23 | 696 ± 132 | 511.52 ± 105 | 0.3 |
| IL-6 | 5.28 ± 1.1 | 2.35 ± 0.36 | 0.02 |
aValues are expressed as Mean ± SD.
5. Discussion
The first major finding of this study was a significant difference in the serum IL-23 levels of the schizophrenia patients and the controls. IL-23 was previously shown to induce the production of IFN-γ, which is important in Th1 responses. IL-23 has also been shown to play a leading role in the activation on NK cells, T-cell production, and regulation of antibody production. Furthermore, studies suggested that IL-23p19 might have an important role in the pathogenesis of MS and experimental autoimmune encephalitis (21, 22). In some schizophrenia patients, a nonspecific generalized immune response involving both T-helper cell type 1 and 2 immune activation has been reported. These last two theories propose that a subgroup of patients with schizophrenia may exhibit features of an autoimmune process, a theory which has been supported by a several investigations (23, 24). The autoimmune hypothesis is also supported by the finding of augmented autoimmune diseases in relatives of schizophrenic patients (25, 26). Studies have repeatedly demonstrated that certain groups of schizophrenic patients show symptoms of an autoimmune process. Increased levels of IgG antibodies have been detected in the CNS of subgroups of schizophrenic patients (27). Elevated antibodies against heat shock protein 60 are one of the interesting observations in schizophrenia, because shows that a process of loss of neuronal protection has occurred in schizophrenia (10).
To our knowledge, the detection of significantly higher IL-23 levels in the serum of schizophrenia patients compared to those of healthy controls has not previously been reported (28). Although our results revealed no significant change in post-treatment IL-23 levels in comparison to pretreatment levels, the antipsychotic treatment led to a reduction of about 26% in IL-23 levels. Therefore, if the sample size or duration of treatment increased for 2 or 3 months, IL-23 serum levels might be significantly decreased compared to those of schizophrenic patients before treatment, and antipsychotic treatment could affect the serum levels of IL-23. The data indicate that excessive/abnormal immune activation may be a characteristic of schizophrenia. Our findings are in agreement with the results of O’Connell et al. (29). Further studies are needed to clarify the relationship between schizophrenia and elevated IL-23 levels and to determine whether there is a correlation between IL-23 levels and the duration of the disease. A number of studies showed that proinflammatory cytokines and neurotrophic factor S100B were elevated in the sera of female patients with schizophrenia but not in male patients in comparison to those of controls (30-32). Further studies in this area would provide useful data to improve our understanding of the pathophysiology of schizophrenia.
The second major finding in this study was the presence of significantly higher levels of IL-6 in the schizophrenia patients compared to those of the control group. Previous studies that detected increased levels of IL-6 and TNF-α system in serum indicated that the underlying mechanism might be related to monocyte and T-cell activation. However, it is not clear whether T-cell activation involves Th1, Th2, or Th17 systems. Several studies showed that IL-6 was a product of stimulated monocytes and the type-2 immune response. Our results are in accordance with those of Schwieler et al. (17) and Frydecka et al. (33).
5.1. Conclusion
Our results support the findings that the immune system is activated in schizophrenic patients. Both serum levels of IL-23 and IL-6 were elevated in the schizophrenia patients compared to those of the healthy controls. These cytokines may represent valuable biomarkers in schizophrenia. Presumably, drugs that inhibit the production of these cytokines could be considered for the treatment of schizophrenia. However, further studies are needed to clarify the role of IL-23 and IL-6 in schizophrenia and their contribution to psychiatric symptoms in schizophrenic patients.