1. Background
Systemic lupus erythematosus is a chronic inflammatory disease characterized by multiple organ damage, mediated by production of autoantibodiesand immune-complex deposition. In the last decade white adipose tissue has been considered to secret adipokines which has been proposed to mediate autoimmunity and inflammation (1). One of the most important adipokines is resistin.
A low molecular weight cystein is rich peptid which is derived from the white adipose tissue (2, 3). With a suggestive role in insulin sensitivity associated with inflammatory conditions (4, 5). The role of resistin in insulin resistant has been described in previous literature but there are lots of controversies in this area of research (6-13). Data has indicated its important role in stimulating role in production of proinflammatory factors such as TNFα and interlukin6 (14, 15) and in inducing some other proinflammatory gens in human adipose tissue (16).
Resistin has been proposed to be associated with markers of disease activity in autoimmune and inflammatory processes such as systemic lupus erythematosus (17-19), rheumatoid arthritis, and Crohn’s disease (19, 20). The object of this study was to determine the relationship between plasma resistin levels and biomarkers of inflammation in patients with SLE and other lupus-disease-related factors.
2. Methods
Seventy three patients with a definit diagnosis of SLE, according to ACR classification criteria were enrolled in the study (21). The controls were selected among the healthy age and sex match individuals. Exclusion criteria were any other inflammatory or rheumatic diseases or infectious processes.The score of disease activity in SLE patients, were determined by SLEDAI; systemic lupus erythematosus disease activity index (22).
Body mass index (BMI) was calculated for all participants. The average of two measurements of the blood pressure, five minutes apart, was recorded at 9 AM.
Venous blood samples were obtained from all of participants after 10 hours over night fasting and the plasma,were stored at-20 degree cellicius.Concentration of plasma resistin were determined byELIZA Bio Vendor kit. The other markers such as ESR, hs-CRP, ANA, anti dsDNA, serum complement level and serum creatinin were compared between two groups. Dose and duration of hydroxychloroquine and prednisolon intake were recorded.
24 hours urine collections were examined for proteinuria which was defined as > 500 mg/24h.
2.1. Statistical Analysis
Statistical analysis was performed using spss17 software. Mann Whitney U test and independent sample t-test were used to analyze the data. Pearman correlation was modeled to studying the association between two variables. P values < 0.05 were considered to be statistically significant.
3. Results
Seventy three lupus patients (8 male, 65 female) and sixty five controls (9 male, 56 female) were enrolled in the analysis. The mean age of lupus patients was 30.55 ± 9.38 year and 89.2%female. The mean age of the control group was 31.80 ± 6.37 year and 86.2% were female. There was no statistically significant difference in age (P ≤ 0.357) and gender (P ≤ 0.606) between two groups.
There was no difference between BMI in those two groups (P = 1.054, t = 0.294) (Table 1). Statistical analysis showed no significant difference in concentration of serum resistin, between patients and controls (6.88 ± 4.53 and 6.74 ± 3.74 ng/mL respectively, P = 0.704).
aStatistically significant.
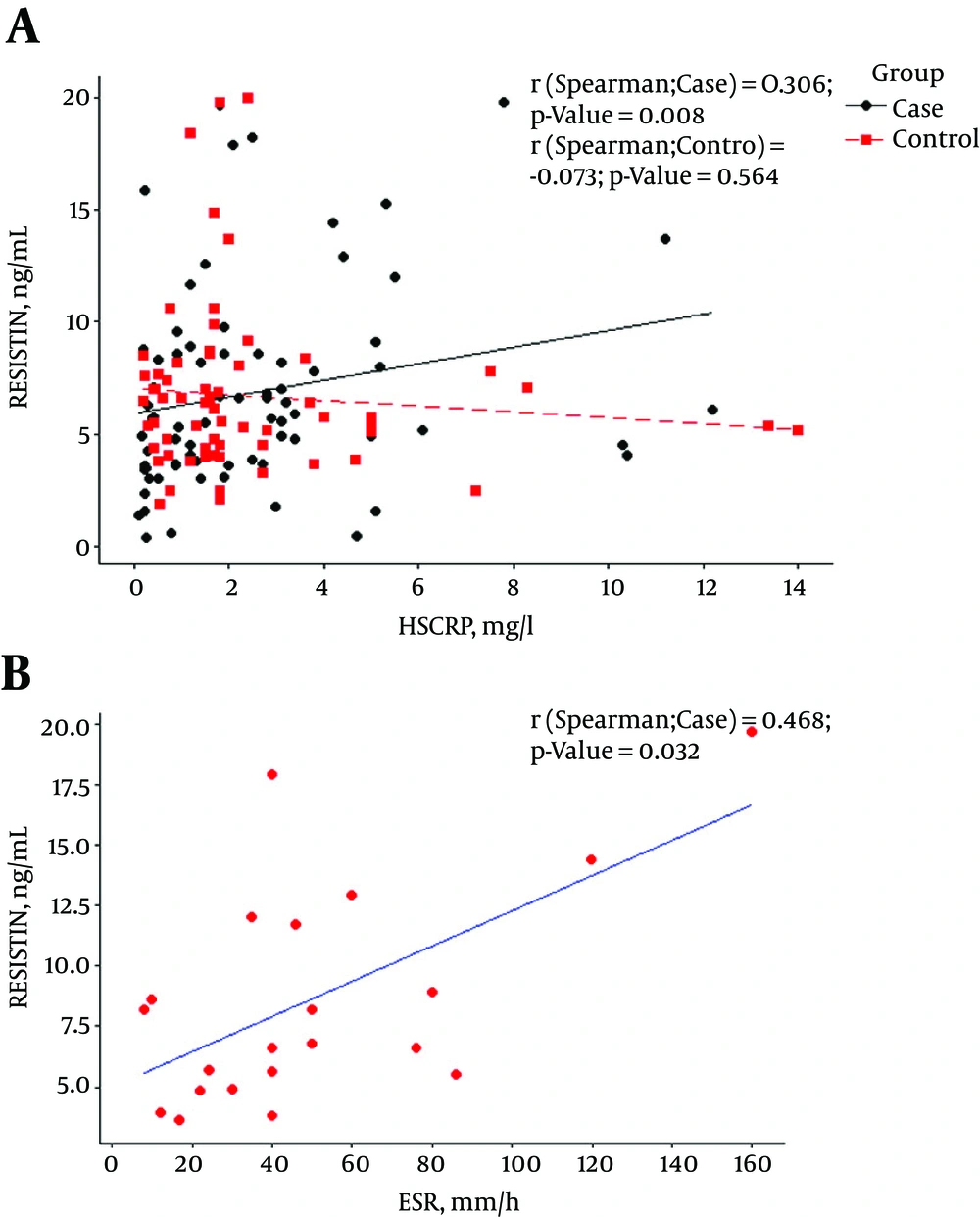
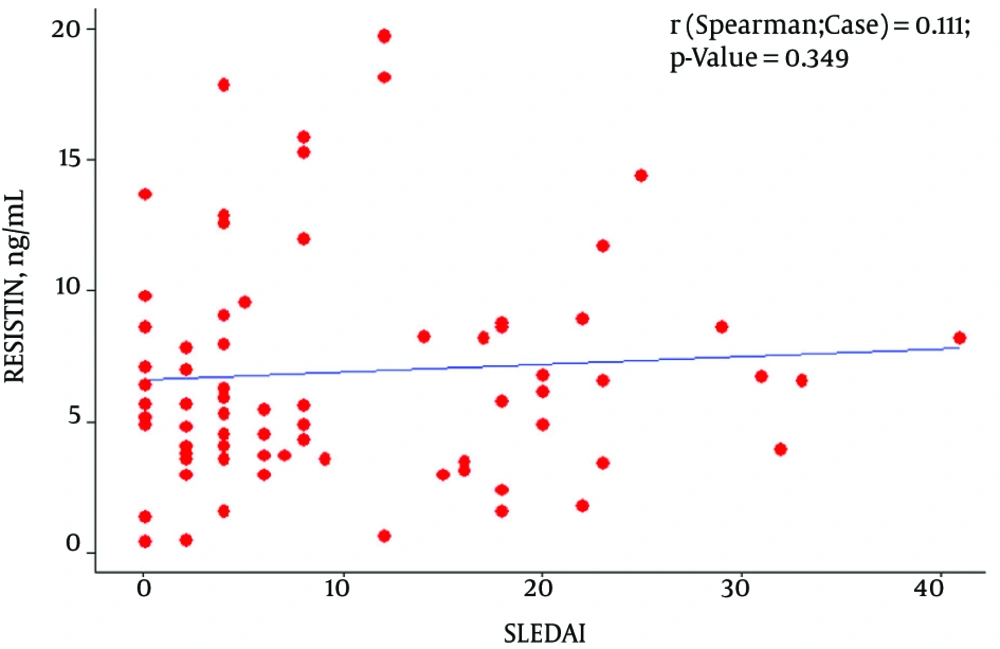
Among SLE patients, level of serum resistin was significantly correlated with CRP and ESR (P ≤ 0.008 and P ≤ 0.037 respectively, Figure 1), but there was no correlation between resistin and the other markers of disease activity (Figure 2) and neither correlation between resistin and Hydroxychloroquine duration (month) (P-value:0.407), prednisolone duration (month) (P-value:0.640) and prednisolone dosage (mg) (P-value:0.188).
Mean serum levels of serum resistin in lupus patients with and without proteinuria was 8.82 ± 5.25 and 6.02 ± 3.92 respectively. Higher serum levels of resistin in patients with proteinuria were found in comparison with normal kidney patients (P = 0.022).
4. Discussion
In this study we did not find any significant difference in concentration of serum resistin in values of SLE patients compared with healthy individuals. This result are in line with previous studies managed by Almehed et al. (17), Vadacca et al. (23) and De Sanctis et al. (24). However, in Liu Lunfei study, resistin in patients with active lupus was significantly higher than patients with low disease activity and it had a positive correlation with SLEDAI (25).
An expected finding of this study was a distinct association between resistin and inflammatory markers such as ESR and hs- CRPin lupus patients. This is concordant with some earlier studies, including Kunnari (26) and Almehed (17) study that found a positive relationship between resistin and inflammatory markers. Among patients with SLE, we found a positive significant association between 24 hours proteinuria and the concentration of serum resistin in lupus patients. These finding are mostly compatible with Almehed study that found a correlation between resistin and nephritis in this population (17).
Patients with SLE have been reported to possess significantly higher levels of serum proinflammatory cytokines; including tumor necrosis factor and interleukin-6 (27). It has been reported that resistin can induce a pro inflammatory state “in vitro” as well as “in vivo” (16). In spite of the numerous recent researches in the field of resistin pathophysiology, little is known about how resistin works in the process of inflammation. It has been demonstrated that proinflammatory mediators such as TNF-a, IL-1b, IL-6, or lipopolysaccharide (LPS) can highly increase the expression of resistin in peripheral blood mononuclear cells (PBMCs), recommending a role for resistin in the process of inflammation (14, 16, 28).
In this cross sectional study, we hypothesized that serum levels of resistin presumably can reflect the level of disease activity according to the SLEDAI in SLE patients. But the present results did not confirm it. However an association between serum values of resistin and inflammatory markers was seen. Elshishtawy found that concerning correlation of serum resistin with other variables, a highly significant positive correlation with SLEDAI exists (29). In another study by Baker et al., they noted that the SLE Disease Activity Index (SLEDAI) was not significantly related to resistin levels in patients with SLE (30).
In a recent study by J. Hutcheson et al., they noted that “the expression of adiponectin and resistin increased in both sera and urine from LN patients, while leptin was increased in LN patient sera, compared to matched controls. Serum resistin, but not urine resistin, was correlated with measures of renal dysfunction in LN. Serum resistin expression may be useful as a marker of renal dysfunction in patients with LN” (31).
In another study performed by Sharifipour et al. in which they examined the urinary biomarker in lupus nephrities, they evaluated the association of urinary lipocalin-2 with lupus nephritis. They found out that in LN patients, urinary lipocalin-2/creatinine significantly correlated with proteinuria (r = 0.68; P = 0.0001) (32). However, it should be noted that, unlike in our study, the level of resistin and its correlation with proteinuria was not evaluated in their research.
Limitation of this study was selection of the SLE patients who were under treatment with a low disease activity, so the results of this study may not be generalized to patients with severe active lupus disease.
4.1. Conclusion
According to the results, presented in this paper and by comparison with the other studies, we can conclude that resistinis associated with inflammatory markers but not it doesn’t have a remarkable association with markers of disease activity such as the SLEDAI.
These studies will provide further beneficial information to design the most appropriate clinical trial to answer the notable questions about potential biologic effects of resistin and the other adipokins in subjects with SLE.