1. Introduction
Klebsiella pneumoniae is a member of the Klebsiella genus of Enterobacteriaceae. It is found in the normal flora of mouth, skin, and intestine. Nowadays, Klebsiella species are known as part of the important nosocomial pathogens. Most common infection caused by Klebsiella spp. is pneumonia, which usually causes bronchopneumonia (1). The rate of mortality is about 50% or more and even by antimicrobial treatment (2).
Electromagnetic irradiations are able to have an influence on bacteria and enhance (3) or inhibit (4) the bacterial growth rate. Extremely low-frequency magnetic fields can cause accumulation of the intracellular minerals such as calcium (5, 6). Calcium ions (Ca2+) are essential in the membrane for ATPases activity that provides energy for efflux pumps and ion channels (7). The intrinsic resistance to antibiotic substances is essential because of the efflux pumps that enables bacteria to stay alive in the presence of these toxic agents (8, 9).
Electromagnetic fields (EMFs) are also able to cause acute and chronic effects on the cells by increasing intracellular free radical levels. The free radicals can damage DNA and develop harmful cellular responses (6, 10). Several studies showed that EMFs could be applied as an antibacterial agent and also utilize as a nonchemical agent for healing or injuring the cells (11-13).
It is well known that physicochemical agents such as EMFs might be directly affected by bacterial growth and the bacteria are able to respond to the environmental stressors (14, 15). Several studies have demonstrated that EMFs can induce changes in bacterial morphology (16), antimicrobial susceptibility (17), proliferation, growth rate (18), and DNA repair (19).
Today, antibiotic resistance of bacteria is increasing due to overuse of antibiotics in chemotherapy, food source, agriculture, and animal husbandry (20-23). Bacterial resistance to one or more antibiotics occurs by various mechanisms (24-26). In this way, EMFs as an environmental agent could influence cellular responses such as antimicrobial susceptibility through different pathways (4, 20, 22, 23, 27). The aim of the present study was to evaluate the antimicrobial susceptibility of K. pneumoniae under 900 MHz radiofrequency radiations condition.
2. Methods
2.1. Antimicrobial Susceptibility Test
In this study, Klebsiella pneumoniae was isolated from the urine culture of the patients and identified in Shahid Faghihi hospital, Shiraz, Iran through conventional biochemical methods and confirmed by API protocol. The pure culture of K. pneumoniae was diluted in Mueller-Hinton Broth and reached 0.5 McFarland turbidity standard to get 1.5 × 108 CFU/mL as the total count (28). The bacterial suspension was spread on Mueller-Hinton agar (MHA-Biolife, Italy) plates and cultured with a set of antibiotics. They were tested by the disk diffusion method (Kirby-Bauer method) according to the clinical and laboratory standards institute guidelines (CLSI, 2013) that was conducted in the medical microbiology of Shiraz University of Medical Sciences. The incubation period was 18 - 24 hours at 35°C, then inhibition zone diameters for each antibiotic were measured.
2.2. Antimicrobial Agents
The antibiotics used were Imipenem (IMI 10 μg), Aztreonam (AZT 30 μg), Cefotaxime (CTX 30 μg), Piperacillin (PIPRA 100 μg), and Ceftriaxone (CTR 30 μg). (ROSCO Diagnostica DK-2630 Taastrup, Denmark). The results of antibiotic susceptibility tests pre and post exposure to 900 MHz radiation produced by RF simulator, were measured and analyzed. The inhibition zone of each antibiotic disk was recorded as mean in millimeter (mm). Three replicate agar plates were used for each regime (17).
2.3. RF Simulator
In this study, all exposures were performed using a GSM (900 MHz) mobile simulator (designed by department of medical physics and biomedical engineering, Shiraz, Iran) operating in the “Talk mode” and simulates the real condition of mobile phone radiation during calling. The radiofrequency (RF) simulator operated on the specific absorption rate (SAR) at the distance of 10 cm of the bacterial suspension (29).
2.4. Outgrowth Curve
The effects of radiofrequency exposure on the growth rates of bacteria were also investigated. The specified concentration of bacterial suspension inoculated in the broth medium precisely and then divided into 2 sets as a non-exposed (control) and the radiofrequency (RF) simulator exposed groups. For estimating the number of bacterial cells in a broth medium, the turbidity of each group was measured using optical density (OD) in 625 nm absorption (30) at different times by a spectrophotometer (UNICO UV-2100).
2.5. Statistical Analysis
All experiments were replicated 3 times for exposed and non-exposed groups. The means were compared using the non-parametric Mann-Whitney and t-test using SPSS 15 (P < 0.05).
3. Results
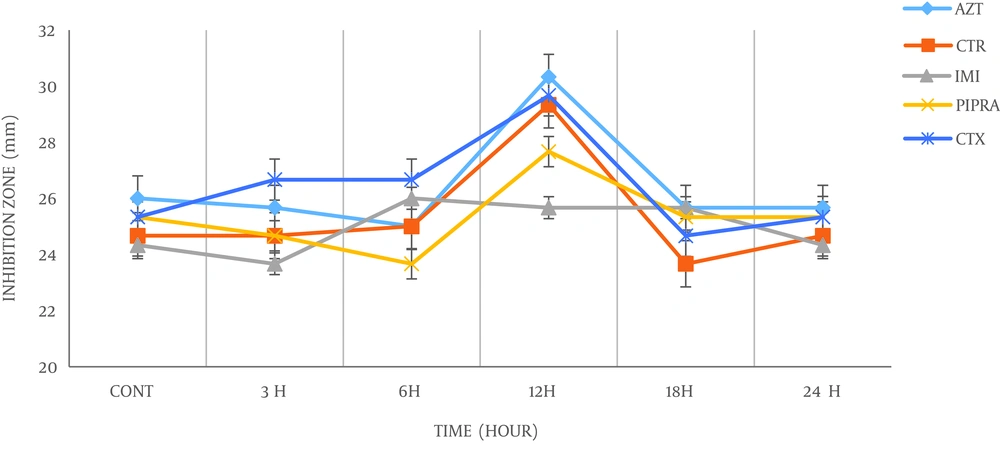
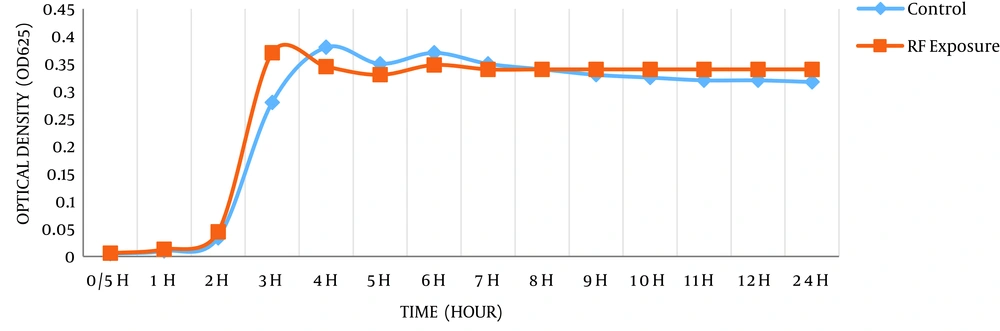
In the current study, antimicrobial susceptibility results of K. pneumoniae to 5 commonly antibiotics after exposure to 900 MHz radiofrequency radiation, for exposed and non-exposed (control) bacteria were summarized in Table 1. Furthermore, the effect of radiofrequency (RF) radiations on the growth rate of the bacteria was evaluated and the results for exposed and non-exposed bacteria were presented in Table 2. During exposure to radiofrequency radiation, the antimicrobial sensitivity of the bacteria was changed, but on the 12th hour of exposure, the sensitivity of the bacteria increased significantly (P < 0.05).
| RF Simulator | ||||
|---|---|---|---|---|
| Exposure Time | Drug | Control | Exposure | P Value |
| 3h | AZT | 26 ± 0 | 25.6 ± 0.58 | 0.2983 |
| CTR | 24.6 ± 0.58 | 24.6 ± 0.58 | 1.0000 | |
| IMI | 24.3 ± 0.58 | 23.6 ± 0.58 | 0.2134 | |
| PIPRA | 25.3 ± 0.58 | 24.6 ± 0.58 | 0.2134 | |
| CTX | 25.3 ± 0.58 | 26.6 ± 0.58 | 0.0516 | |
| 6h | AZT | 26 ± 0 | 25 ± 0 | 0.0001* |
| CTR | 24.6 ± 0.58 | 25 ± 0 | 0.2983 | |
| IMI | 24.3 ± 0.58 | 26 ± 0 | 0.0071* | |
| PIPRA | 25.3 ± 0.58 | 23.6 ± 0.58 | 0.0230* | |
| CTX | 25.3 ± 0.58 | 26.6 ± 0.58 | 0.0516 | |
| 12h | AZT | 26 ± 0 | 30.3 ± 0.58 | 0.0002* |
| CTR | 24.6 ± 0.58 | 29.3 ± 0.58 | 0.0006* | |
| IMI | 24.3 ± 0.58 | 25.6 ± 0.58 | 0.0516 | |
| PIPRA | 25.3 ± 0.58 | 27.6 ± 0.58 | 0.0083* | |
| CTX | 25.3 ± 0.58 | 29.6 ± 0.58 | 0.0008* | |
| 18h | AZT | 26 ± 0 | 25.6 ± 0.58 | 0.2983 |
| CTR | 24.6 ± 0.58 | 23.6 ± 0.58 | 0.1023 | |
| IMI | 24.3 ± 0.58 | 25.6 ± 0.58 | 0.0516 | |
| PIPRA | 25.3 ± 0.58 | 25.3 ± 0.58 | 1.0000 | |
| CTX | 25.3 ± 0.58 | 24.6 ± 0.58 | 0.2134 | |
| 24h | AZT | 26 ± 0 | 25.6 ± 0.58 | 0.2983 |
| CTR | 24.6 ± 0.58 | 24.6 ± 0.58 | 1.0000 | |
| IMI | 24.3 ± 0.58 | 24.3 ± 0.58 | 1.0000 | |
| PIPRA | 25.3 ± 0.58 | 25.3 ± 0.58 | 1.0000 | |
| CTX | 25.3 ± 0.58 | 25.3 ± 0.58 | 1.0000 | |
Abbreviations: AZT, Aztreonam; CTR, Ceftriaxone; CTX, Cefotaxime; IMI, Imipenem; PIPRA, Piperacillin.
aValues are expressed as mean ± SD.
| Experimental Results | |||
|---|---|---|---|
| Time | K. pneumoniae | ||
| OD625 | |||
| Control | Exposure | P Value | |
| 0h | 0.005 ± 0.001 | 0.006 ± 0.0005 | 0.196 |
| 1h | 0.01 ± 0.001 | 0.013 ± 0.001 | 0.0213* |
| 2h | 0.03 ± 0.001 | 0.04 ± 0.001 | 0.0003* |
| 3h | 0.28 ± 0.001 | 0.37 ± 0.002 | 0.0001* |
| 4h | 0.38 ± 0.001 | 0.34 ± 0.001 | 0.0001* |
| 5h | 0.35 ± 0.002 | 0.33 ± 0.002 | 0.0003* |
| 6h | 0.37 ± 0.002 | 0.34 ± 0.001 | 0.0001* |
| 7h | 0.35 ± 0.002 | 0.34 ± 0.002 | 0.0036* |
| 8h | 0.34 ± 0.001 | 0.34 ± 0.001 | 0.9999 |
| 9h | 0.33 ± 0.003 | 0.34 ± 0.001 | 0.0054* |
| 10h | 0.32 ± 0.001 | 0.34 ± 0.002 | 0.0001* |
| 11h | 0.32 ± 0.003 | 0.34 ± 0.001 | 0.0004* |
| 12h | 0.32 ± 0.003 | 0.34 ± 0.002 | 0.0007* |
| 24h | 0.317 ± 0.001 | 0.34 ± 0.003 | 0.0002* |
Abbreviation:OD, optical density.
According to Table 1, it is clear that 900 MHz radiation has altered the antimicrobial susceptibility of the bacteria, which may be due to changes in their physicochemical properties. Hence, inhibition zone diameters of the pre and post exposed bacteria, for each antibiotic were measured and showed variations at different time exposure. As shown in Figure 1, there were no significant changes in sensitivity of the bacteria after 3rd and 6th hour, however, after exposure to the 12th hour, a maximum significant rise in the antibacterial sensitivity of the bacteria was observed. At the 18th and 24th hour exposure, the sensitivity of the bacteria to all antibiotics was decreased and bacteria tend to return to the early condition. Also, the effects of 900 MHz radiofrequency radiation on the growth rate of bacteria was carried out (Figure 2). Based on the bacteria that were exposed to radiation showed faster growth rate in exponential phase compared to bacteria in non-exposed groups. Moreover, the time to reach the logarithmic phase in the growth curve of the bacteria was faster in exposed groups.
4. Discussion
Results of the current study are in accordance with the previous study and similar studies on the effects of radiofrequency on the antimicrobial susceptibility of the K. pneumoniae after exposure to 2.4 GHz Wi-Fi radiofrequency radiation and supports the "window" theory concept (17, 31-36).
Based on this theory, when the irradiation level is within the window (between the lower and upper levels of the window), stimulatory effects of ionizing or non-ionizing radiation can be detected. Hence, the response of bacteria and other microorganisms to any environmental stressors can be determined by different field parameters such as the magnitude of the dose and dose rate. By comparison between this study and our previous study on K. pneumoniae response to radiofrequency radiation, the same pattern was repeated and showed a rise in antibacterial sensitivity within the window. However, for Wi-Fi exposure, this significant change was observed at 4.5 hours and for the RF simulator at the 12th hour. Many studies have demonstrated that the bacterial response to electromagnetic fields is dependent on several factors including: intensity, frequency, duration of exposure, and other physicochemical properties of the fields (22, 37, 38). As mentioned, the frequency of Wi-Fi router was 2.4 GHz and RF simulator was 900 MHz, maximum bacterial response to this radiation occurred at different times, which was related to their frequencies. Consequently, for Wi-Fi with higher frequency, this response was observed at 4.5 hours and for RF simulator with lower frequency, the same response was observed at the 12th hour.
We have also examined the effect of radiofrequency radiation on the growth rate of bacteria. As shown in Figure 2, during the investigated time period, K. pneumoniae showed a significant growth rate after exposure. However, several studies had indicated a fall in growth rate of bacteria depending on the field parameters for instance frequency, intensity, the magnitude of the field and exposure duration (20, 23). In another study on E. coli, growth rate decrease was more visible by 53 GHz radiation. Therefore, antibiotic susceptibility changes as a result of electromagnetic radiations (20). In one study (39) that demonstrated Low energy, low frequency radiation enhances the growth rate of microorganisms, although high-energy, high-frequency radiation kills the microorganisms.
The effects of electromagnetic fields on the biological systems are irrefutable. The modifications caused by irradiation have been usually considered as a harmful public concern. Hence, research on the probability of electromagnetic fields could be exploited for advantageous purposes.
Our findings of this study, on the antibacterial susceptibility of K. pneumoniae before and after exposure to 900 MHz RF radiation should be better considered in the treatment of the patients who suffer from the Klebsiella infections after additional investigations. Therefore, understanding the molecular mechanism involved in this response is very important. Previous studies on the bacterial sensitivity affected by electromagnetic fields were carried out and different mechanisms described this phenomenon:
a) Surface charges and membrane potential. Changes in membrane potential and surface charge, make disruption in the electron transport system and energy generation by proton motive force of bacteria would be impaired (40-42) and can increase sensitivity to antibiotics (43). Since membrane potential was crucial for bacterial binary fusion, it can be interpreted that exposure to EMFs influences the behavior of bacteria in the environment (44).
b) Increased efficacy of the antibiotics can be described as a result of electromagnetic field interactions with water molecules in the aqueous environment (45). It is obvious that the electromagnetic field changes physicochemical properties and hydration ability of water molecules and solubility of the antibiotics in the surrounding area was changed (20, 23).
c) One of the factors that can influence antibacterial sensitivity is the cell wall structure of bacteria and peptidoglycan (PG) nature (3, 46, 47). In gram-positive bacteria, cell wall thickness is greater than that of gram negatives.
d) It was demonstrated that electromagnetic field induces permeability of the bacteria and cells remained permeable after exposure to an electromagnetic field at least for 9 minutes especially with 18 GHz (47).
e) Efflux pumps and ion channels located in the cell membrane play an important role in antibiotic uptake by the cell. Electromagnetic fields are capable of changing the channels and pumps and duration of opening time will increase (17, 48, 49).
f) The last factor that can have an influence on the sensitivity of bacteria in the electromagnetic condition is the antibiotic structure. Charge, size, or hydrophilicity of the antibiotics can alter after being exposed to electromagnetic fields (50).
4.1. Conclusions
The bacteria were capable of responding to environmental stresses by activating some specific systems such as ion channels, change via the membrane, and DNA repair system. Considering these results, we believed that mobile exposure can serve as physical methods to change the antibacterial susceptibility of the microorganisms. In this light, K. pneumoniae responds to 900 MHz radiofrequency radiation exposure, variously and significant changes were observed at the 12th hour of exposure. Considering the importance of infections, especially caused by K. pneumoniae, experiments on different bacterial strains with various electromagnetic fields should be performed in the future to better clarify these uncertainties.
4.2. Suggestions
In order to extend the findings of this study to the other population, further studies on the molecular mechanisms of the bacterial responses and working on the several pathogenic, gram positive and negative bacteria, different exposure sources and time exposure will be suggested.