1. Introduction
Diabetes, as a global epidemic disorder, is introduced as the seventh leading cause of death in the world. This disorder is classified into two major groups, based on the reduction in insulin secretion or insulin malfunction: type I and type 2 diabetes mellitus (1-4). There has been an alarming increase in the prevalence of diabetes, especially type II diabetes, in recent years worldwide. The prevalence of type II diabetes is also increasing in Iran due to lifestyle changes, reduced physical activity, poor nutrition, and obesity (5, 6).
Uncontrolled type II diabetes mellitus is associated with the innate immune system (eg, macrophages, mast cells, and eosinophils) and can lead to chronic inflammation (7). Mechanisms, such as oxidative stress, endoplasmic reticulum stress, hypoxia, amyloid and lipid deposition, lipotoxicity, and glucotoxicity, can also promote inflammation. On the other hand, there is a connection between inflammation in adipose tissues and systemic insulin resistance (8).
Studies have shown that macrophages migrate to adipose tissues, liver, and muscles in diabetic and obese people and produce pro-inflammatory factors, such as nitric oxide, reactive oxygen species, prostaglandin E2, tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6, which induce inflammation (9). Furthermore, diabetic patients are sensitive to infectious diseases (10). Inadequate glucose control and its relationship with inflammation in diabetes can lead to severe complications such as atherosclerosis, lung function disorders, and cardiovascular diseases (7).
Recently, researchers have identified a higher level of inflammatory cytokines in patients with type II diabetes, compared with the normal population. Inflammation was found to potentiate the elevation in insulin resistance (11). Moreover, previous studies have shown that inflammatory cytokines are potentially procarcinogenic and suggested a positive association between inflammation and increased risk of several diseases such as colorectal adenoma (12).
Among all inflammatory mediators, IL-6 is a multifunctional cytokine, which is produced and secreted by various cells, such as adipocytes, endothelial cells, smooth muscles, fibroblasts, lymphocytes, and macrophages. Previous studies have shown that IL-6 level in disorders, including obesity, diabetes, and cardiovascular diseases, is increased (9). The role of IL-6 level in insulin resistance, occurrence of type II diabetes, and consequent complications (eg, retinopathy and endothelial cell dysfunction) has been confirmed in the literature (13). Therefore, it seems that IL-6 level is a therapeutic target for overcoming the insulin resistance phenomenon (14).
Among therapeutic agents, thiazolidinediones are known to increase insulin sensitivity and decrease blood glucose level. These chemical agents can eventually increase vascular function and reduce impaired lipid and glucose metabolism, as well as inflammation (13, 14). Nevertheless, lactic acidosis, for instance, is the most serious complication of biguanides (15).
Today, in traditional medicine, more than 1200 plants with anti-diabetic activities have been identified for the treatment of diabetes. Plant-based therapies are preferred, given their cost-effectiveness and lower toxicity in comparison with synthetic agents (16). Overall, attention to herbal medicines with anti-diabetic properties has increased. One of the medicinal plants with anti-diabetic effects is Stevia rebaudina Bertoni, which is one of 154 members of the genus Stevia, producing sweet steviol glycoside (17, 18).
It has been reported that Stevia rebaudiana can maintain the blood glucose level via glucose tolerance enhancement in diabetic rats (19). Stevia could also cause hypoglycemia in diabetic patients through decreasing glycogenolysis and gluconeogenesis and absorbing glucose in the duodenum. In addition, anti-hyperglycemic and anti-oxidative properties of stevia and its glycoside have been detected in several tissues (eg, kidney, liver, and pancreas tissues) (20-22).
In a recent study, we showed that stevia affects pancreatic tissues by elevating the insulin level and exerts beneficial anti-hyperglycemic effects through a PPARγ-dependent mechanism and antioxidant activities (22). Furthermore, several hypotheses have been proposed to explain how stevioside, a derivative of stevia, causes such a significant decline in blood glucose level. Also, various studies have shown that stevioside can reduce inflammation by inhibiting the synthesis of pro-inflammatory cytokines (23). In fact, stevioside and steviol both reduce inflammation by activating and increasing the expression of IκBα gene (NF-κB localization inhibitor) (24).
To the best of our knowledge, the exact anti-inflammatory and immunomodulatory effects of the whole aquatic extract of stevia are not fully understood. Therefore, in the current research, we aimed to determine whether stevia can improve IL-6 level in streptozotocin-nicotinamide (STZ-NA)-induced diabetic rats.
2. Methods
2.1. Preparation of the Aquatic Extract of Stevia Leaf
Stevia was provided by Golsaran company (Rasht, Iran) during March 10 - 29. The harvest time for stevia is twice per year, once in the middle of July and once in the middle of September. After harvesting, all the leaves were washed and then dried at < 50°C. The dried leaves were subjected to size reduction to coarse powder. Subsequently, the powder was extracted in a Soxhlet apparatus and evaporated to dryness under reduced pressure in a vacuum rotary evaporator. The extract was air-dried until a solid to a semi-solid mass was obtained.
Then, 100 g of the powder extract suspension was prepared in 1200 mL of distilled water and kept in dark for 24 hours. Following that, the solution was filtered and the extract was evaporated into the rotary or a water bath at a temperature range of 40 - 50°C. To ensure that the extraction has no humidity, it was placed in a vacuum desiccator for 24 hours; eventually, the efficacy was calculated.
2.2. Animal Experiments
All the experiments on animals were approved by the Ethics Committee of Shiraz University of Medical Sciences. Adult male Wistar rats (200 - 250 g) were born and bred at the endocrine and metabolism research center of Namazi Hospital. In the present study, 5 groups of rats were studied, each including 11 rats. They were placed in 5 cages and were fed a rat chow diet (Pars Dam, Tehran, Iran) with water.
Type II diabetes was induced with an intraperitoneal injection of NA (110 mg/kg) at 15 minutes before the intraperitoneal injection of STZ (60 mg/kg). Following diabetes induction (after 7 days), blood was drawn from the tail vein of experimental rats to determine their fasting blood sugar (FBS) level. Rats with an FBS level of ≥ 300 mg/dL developed diabetic symptoms, such as weight loss, hyperplasia, polydipsia, and polyuria. These animals were considered diabetic and were selected for treatment.
The cohort of rats in the present study included the following 5 groups, each containing 11 rats: (1) normoglycemic group, receiving 1 mL of water; (2) diabetic control group, receiving 1 mL of water; (3) and (4) diabetic groups treated with the aquatic extract of stevia (400 mg/kg) and metformin (500 mg/kg), respectively; and (5) healthy group treated with the aquatic extract of stevia (400 mg/kg). All the groups received treatment through gavage in a single dose every morning for a period of 30 days.
At the end of the experiment, the rats were sacrificed and blood was collected from their heart. Then, the blood samples were centrifuged at 3000 rpm for 10 minutes. Serum was immediately isolated and maintained at a temperature of -80°C for further analysis.
2.3. IL-6 Level Measurement
The collected serum samples from all the groups were diluted in accordance with the manufacturer’s instructions in the kit. Then, IL-6 level was measured by the enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen Corporation, Camarillo, CA 39012, USA). The mouse IL-6 ELISA includes a solid phase sandwich ELISA, which contains a monoclonal antibody specific for rat IL-6, coated on the plate.
2.4. Glucose Measurement
The serum glucose level was measured after 30 days of treatment with diagnostic colorimetric kits (BioSystem, Spain), using Prestige Instrument (Hitachi, Japan).
2.5. Statistical Analysis
The normal distribution of the data was evaluated, using one-sample Kolmogorov-Smirnov test. Distribution of the data was normal and variances were homogeneous. The results were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed, using one-way analysis of variance (ANOVA), followed by least significant difference (LSD) post-hoc multiple comparison test, using SPSS version 16 (SPSS, Chicago, IL, USA). P value less than 0.05 was considered statistically significant.
3. Results
3.1. Effect of the Aquatic Extract of Stevia rebaudiana Bertoni on Serum Glucose and IL-6 Levels
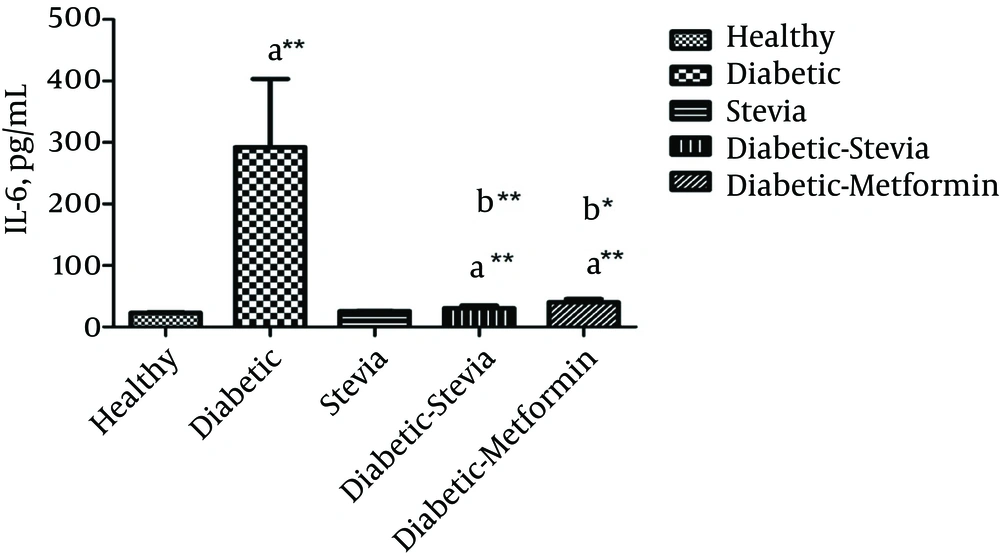
Figure 1 and Table 1 represent the effects of 400 mg/kg of the aquatic extract of stevia on blood glucose and IL-6 levels in 5 experimental groups. In addition, Table 1 shows the glucose level and weight of rats at baseline. The present results showed that IL-6 (P = 0.001) and FBS (P < 0.001) levels significantly increased in all diabetic groups, compared with healthy rats. Unexpectedly, treatment with stevia or metformin could significantly reduce FBS (P < 0.001), as well as IL-6 level (P = 0.001), compared to the diabetic group. However, metformin (500 mg/kg) was found to be more effective than the aquatic extract of stevia (400 mg/kg) in reducing the glucose level.
| Parameters | NC | DC | Diabetic Stevia | Diabetic Metformin | Healthy Stevia |
|---|---|---|---|---|---|
| FBS, mg/dL at baseline | 82.7 ± 3.6 | 81.83 ± 3.28 | 92.2 ± 2.5 | 83.6 ± 1.6 | 78.5 ± 2.39 |
| Body weight, g at baseline | 220.2 ± 3.4 | 242.2 ± 4.2 | 246.8 ± 2.6 | 240.5 ± 3.1 | 231 ± 2.4 |
| FBS, mg/dL after treatment | 77.5 ± 3.8 | 509.8 ± 18.2b | 233.3 ± 61.4c | 89.1 ± 10.5c | 74.8 ± 3.03c |
| IL-6, pg/dL after treatment | 23.2 ± 0.66 | 292.2 ± 10.8b | 30.6 ± 4.1c | 40 ± 5.8c | 25.5 ± 0.62c |
The Effect of Stevia on FBS and IL-6 Levels in STZ-NA-Induced Diabetic Wistar Rats after 4 Weeks of Treatment (FBS Level and Weight at Baseline)a
Based on the findings, there was no significant difference in IL-6 level between the healthy group and rats treated with either stevia or metformin. In addition, there was no significant difference in IL-6 or FBS level between healthy rats and the healthy group treated with stevia.
4. Discussion
Diabetes mellitus is known as a multifactorial metabolic disorder worldwide (1, 25). Due to the heterogeneous nature of this disease, there is an urgent need to find effective and appropriate therapies. Recently, researchers have shown that herbal remedies can help improve diabetic complications (26). There are several well-known plants with hypoglycemic effects, such as Teucrium polium, Smallanthus sonchifolius (yacon), Psacalium peltatum, and Cucurbita ficifolia (27-29). Among these plants, Stevia rebaudiana Bertoni is a natural sweetener for individuals with a carbohydrate–controlled diet.
The current study, similar to our previous research (22), showed that treatment of type I diabetic rats with the aquatic extract of stevia (400 mg/kg) for 30 days significantly reduced the blood glucose level. In the present study, STZ-NA injection induced a significant increase in blood glucose level. Earlier injection of NA into pre-diabetic rats promoted β-cell protection against STZ and produced a model of type II diabetes (30). In this regard, Kudelski et al. showed that intraperitoneal injection of NA in rats leads to an increase in nicotinamide adenine dinucleotide (NAD+) in tissues, which in turn hinders STZ-induced β-cell destruction (30).
According to previous studies, stevia derivatives such as stevioside reduce the blood glucose level. In a similar study in 2000, Jeppesen et al. showed that stevia derivatives by affecting pancreatic β-cells increase the insulin level and reduce blood glucose (31). As revealed by other researchers, the anti-diabetic effect of stevia and its glycoside might be exerted through the stimulation of peripheral glucose utilization or enhancement of glycolytic and glycogenic processes, concomitant with the decline in glycogenolysis and gluconeogenesis and glucose absorption in the duodenum (31-39).
It seems that the beneficial effect of stevoside on STZ-NA-induced diabetic rats might be due to its impact on gluconeogenesis. Chen et al. showed that stevioside can regulate the blood glucose level not only by enhancing insulin secretion, but also by slowing down gluconeogenesis through reducing phosphoenolpyruvate carboxykinase gene expression in the rat liver, which in turn regulates the blood glucose level (35).
Moreover, our previous study showed that stevia affects pancreatic tissues by elevating the insulin level and exerts beneficial anti-hyperglycemic effects through a PPARγ-dependent mechanism and antioxidant activities (22). Therefore, the aquatic extract of stevia (eg, stevioside) can decrease glucose level in rats with diabetes, induced by STZ alone or STZ-NA.
In line with our findings, Satheesh and Pari showed that blood glucose level significantly reduces in diabetic rats treated with metformin (500 mg/kg/day), as the positive control group (40). However, metformin (500 mg/kg) was more effective than the aquatic extract of stevia (400 mg/kg) in lowering the glucose level. To the best of our knowledge, no previous study has compared stevia with metformin with respect to their reducing effects on glucose level.
Overall, it is essential to design in vivo studies and clinical trials in order to gain a better insight into the anti-diabetic activity of stevia. The low dose of stevia compared to metformin could limit its effectiveness; therefore, this factor should be considered in future studies. In fact, in our previous study, a higher dose of stevia could induce better effects in comparison with 10 mg/kg of pioglitazone (22).
A similar observation was also recorded in our previous study (22), showing that stevia does not cause any side-effects regarding glucose level in healthy people, and no hypoglycemia was reported in healthy rats. On the other hand, numerous reports have demonstrated that inflammation, as an important defense mechanism, occurs in diabetic patients (41), which consequently leads to insulin resistance and initiates type II diabetes (42).
In the current study, the serum level of IL-6 increased with the onset of diabetes in rats. The rise in IL-6 level indicated that diabetes induction in rats leads to the onset of inflammation and increases the release of IL-6, as an inflammatory cytokine. The present findings were in consistence with previous studies, which showed that diabetes could induce inflammation. Furthermore, Haidari et al. demonstrated that high plasma level of inflammatory cytokines, such as TNF-α and IL-6, is associated with the development of insulin resistance and type II diabetes mellitus (6).
The present results showed that the serum level of IL-6 in mice, treated with the aquatic extract of stevia (for 30 days), significantly decreased, compared to the diabetic control group. The present findings are in congruence with previous research, which showed that inflammatory cytokines, such as IL-6 and TNF-α, decrease in rats treated with a high concentration of rebaudioside A (a stevia derivative) (43).
Moreover, the results reported by Fengyang et al. in 2010 showed that stevioside significantly inhibits the activity of NF-κB and IκB and reduces the secretion of inflammatory factors, such as IL-6, TNF-α, and IL-1β in RAW2647 cells (9). Another study also examined the anti-inflammatory effects of stevioside and steviol, as stevia derivatives, and showed that they could reduce the expression of IL-6, IL-1β, and IL-10 genes (24).
Moreover, caffeic acid and ellagic acid (phenolic acids), which exist naturally in many plants, such as carrot, tomato, strawberry, and blueberry, can significantly inhibit the mRNA expression of inflammatory cytokines (including IL- 6, TNF-α, and IL-1β) in diabetic mice (44). The aquatic extract of stevia (eg, stevioside) and metformin could reduce the level of IL-6. Both stevia and metformin act through similar mechanisms. In a previous study, serum IL-6 level in polycystic ovary syndrome (PCOS) patients was influenced by metformin. In this study, most women with PCOS suffered from insulin resistance, hyperinsulinemia, and elevated serum IL-6 level (45).
Based on the findings, the aquatic extract of stevia could reduce IL-6 level more than metformin. In the healthy group treated with stevia for 30 days, no significant difference was observed in the serum level of IL-6, compared with the healthy control group. Therefore, the present results showed that the aquatic extract of stevia, similar to its derivatives, could decrease the serum level of IL-6, as an inflammatory mediator in STZ-NA-induced diabetic cases.
4.1. Conclusions
Based on the findings, inflammation might be ameliorated using the whole aquatic extract of stevia, as an anti-diabetic compound. Therefore, IL-6 could be considered as an indicator of insulin resistance, while stevia could lead to reduced IL-6 level and thereby decrease insulin resistance in diabetic patients.