1. Context
Xanthelasmas, also known as “xanthomas” and “lipid islands” are yellow plaque-like lesions characterized by the presence of lipid-containing histiocytes. They can be found on the skin and in mucosal layers. The stomach is the most common location of xanthomas in the gastrointestinal (GI) tract (76%) (1).
Gastric xanthoma (GX) was first defined as “lipid-laden macrophages in the gastric mucosa” by Orth in 1887 cited in Khachaturian et al. (2). Since then, no clinical significance has been defined, yet its association with gastric carcinoma was highlighted. In this review, we discussed the clinical, endoscopic, histopathologic features of GX and its association with gastric and non-gastric diseases.
2. Evidence Acquisition
A search of the following databases was conducted; PubMed and Google Scholar between 1965 and 2015. Keywords such as “xanthoma”, “xanthalesma”, “gastric lesion”, “endoscopic findings”, and “foamy histiocytes” were used. Human and animal studies, case reports and clinical trials that investigated and reported on GX were included. English language titles and abstracts of papers were screened and relevant papers were selected. Next, full texts of relevant papers were read and findings were rescreened. Final results were recorded.
3. Results
3.1. Epidemiology
The reported incidence of GX was quite variable: it ranged from 0.018% to 7% in endoscopy series and was as high as 58% in an autopsy series (3-6). Its prevalence was low in the west; however, for unknown reasons, possibly related to the high prevalence of chronic gastritis, these lesions were common in Asia (7).
Gastric xanthoma can be seen in people of all age; however the incidence of GX increases with age. The mean age has been reported as 60 years (1, 8). However, Collins et al. (9) reported on a case of GX who was as young as two years old and Halabi et al. (10) reported on a three-year-old boy with multiple GX. Gastric xanthoma was found at similar rates in males and females, however some researchers have reported a moderate predominance in males compared to females (male: female = 3.3: 1) (3).
3.2. Pathogenesis
Macrophages are normally found in the lamina propria of the gut and they serve the first line defense mechanism of the mucosa against harmful pathogens as part of the innate immune system (11). In the event of damage of the intestinal epithelium (e.g. infection and inflammatory bowel disease), circulating monocytes join the resident macrophages of the gut and actively pursue the invading pathogens through phagocytosis (12). The foamy cells, which are large cells with foamy cytoplasm, occur by accumulation of endogenous materials in the macrophages. Lipid content of the foamy cells in xanthoma is believed to come from lipids derived from broken cell membranes related to mucosal injury (13).
3.3. Clinical Features
Gastric xanthoma is generally asymptomatic, and the complaints of symptomatic patients are unlikely to be related to GX (1). Because the formation of gastric xanthoma appears to be related to healing processes in response to tissue damage, it is more commonly found in patients who are subject to mucosal damage. Thus, concomitant clinical situations can be found with GX.
3.3.1. Hyperplastic Polyps
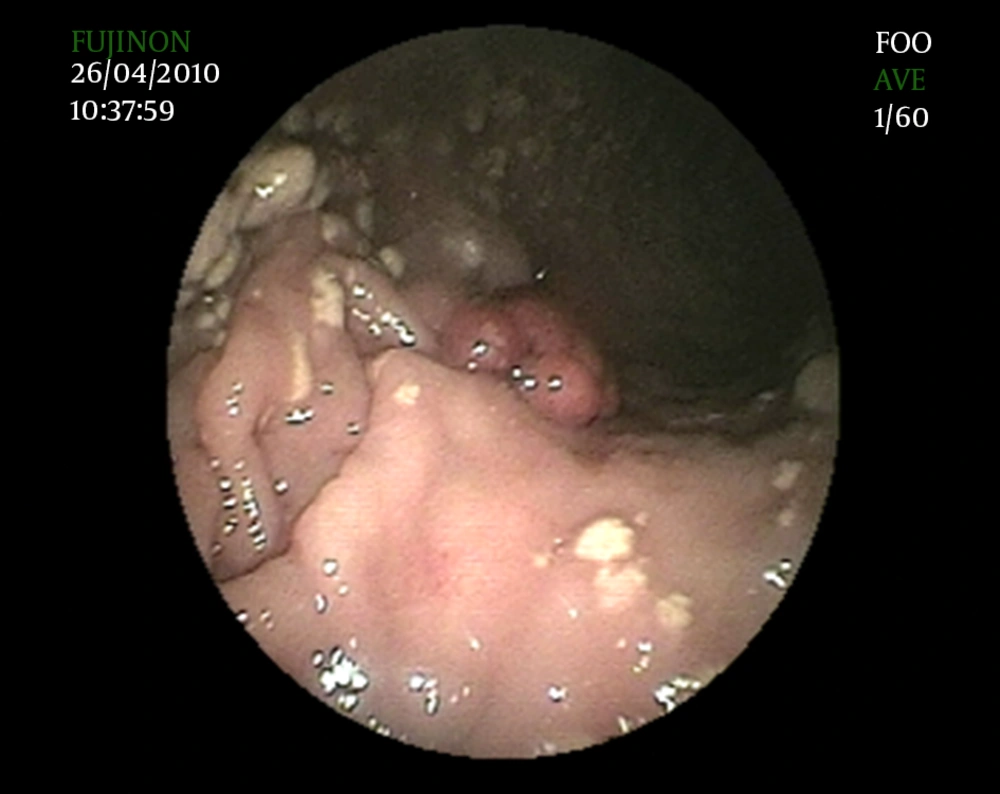
In 1989, Lin et al. first reported on combined lesions showing features of GX and hyperplastic polyps (14). In a study conducted on the current spectrum of gastric polyps, Carmack et al. (7) reported that 10.3% of 154 cases with GX had hyperplastic polyps. We also observed coexistence of multiple GXs and hyperplastic polyp in an unreported case (Figure 1). Carmack et al. (7) reported that hyperplastic polyps with GX were mostly < 3 mm in size, and were found near the site of mucosal repair. The frequency of GX relative to other polyps was reported as 0.3 to 3.9%. Although the etiopathogenesis of GX and hyperplastic polyp and the coexistence of these two lesions are unclear, it has been suggested that they occur as an inflammatory response to focal mucosal damage (15).
3.3.2. Helicobacter pylori (H. pylori)
An association between GX and H. pylori infection has been suggested by previous studies. This relationship can also result from conjunction with gastric injury. Isomoto et al. (16) showed that the prevalence of H. pylori infection was significantly higher in patients with GX compared to patients without GX (94% versus 72%). Helicobacter pylori antigens were identified in 69 of 145 gastric biopsy specimens with GX (48 %) (17). It has been proposed that a proportion of GX may be provoked by H. pylori infection (16, 17).
3.3.3. Atrophy and Intestinal Metaplasia
Patients with GX had a significantly more severe degree of endoscopic mucosal atrophic changes than controls (16). Some studies have reported on the association of GX with atrophic gastritis and intestinal metaplasia (18).
3.3.4. Alkaline Reflux Gastritis
Bile reflux is also associated with the presence of GX. The incidence of GX was shown to increase, after gastric surgery, related to bile reflux. Twenty-three years after gastric surgery, the incidence of gastric xanthomas has reached up to 60%. Intestinal metaplasia with bile reflux has been shown to increase cellular lipid transport (19). Another rare lesion, known as xanthogranulomatous gastritis (XGG), is also believed to be a bile-reflux induced pathogenesis. xanthogranulomatous gastritis has similar histopathological findings as XG yet it has rapidly enlarging submucosal nodules in the stomach (20).
3.3.5. Gastric Cancer
GX is also associated with gastric malignity. Muraoka et al. (21) reported on a patient with early gastric cancer with proliferation of xanthoma cells, and Luk et al. (22) reported on a case of clear-cell carcinoid tumor of the stomach, which was similar to GX in relation to endoscopic and microscopic findings. Sekikawa et al. (23) observed 50 (47.6%) GX cases within 105 patients with gastric cancer and they demonstrated that the presence of GX was significantly associated with the presence of gastric cancer in age/sex/atrophy matched analysis. They proposed that GX may serve as a warning sign for the presence of gastric cancer. This relationship may be explained by gastric damage. Gastric injury and gastric resection carry an increased risk for both gastric cancer and GX. Chronic gastritis is thought to be involved in gastric glandular atrophy and intestinal metaplasia sequence, which are considered as precursors of gastric cancer and GX.
3.3.6. Hyperlipidemia
Although chemical analysis has shown that the foamy cells contain cholesterol with or without neutral fat, no correlation between GX and hyperlipidemia has been indicated (24). However, some investigators have reported rare cases accompanied with hyperlipidemia (19); two cases of GXs have been reported in the literature in severe cholestasis (19). In both cases (one with acute cholestasis and one with chronic cholestasis), GXs disappeared with the resolution of the cholestasis. Researchers have suggested that transient elevated serum lipids may induce the formation of GX and may disappear with resolution of cholestasis. Katsu et al. (25) observed experimental GX formation in rabbits, which had been treated with chlormadinon acetate undergoing cholesterol feeding and had high serum cholesterol levels. This suggests a relationship between hypercholesterolemia and GX.
3.3.7. Other Xanthomatosis
It can be complicated with other GI xanthomas principally colonic xanthomas in 13.9% of cases (26).
3.3.8. Xanthoma Disseminatum
This is one of the several heterogeneous conditions caused by the proliferation of non-X histiocytic cells. It is characterized by widespread mucocutaneous xanthomas. Rarely, however, it may be accompanied by systemic involvement. The involvement of the mucous membranes may be observed in 30% - 50% of cases, including gastrointestinal tract (27).
3.4. Diagnosis
3.4.1. Endoscopically
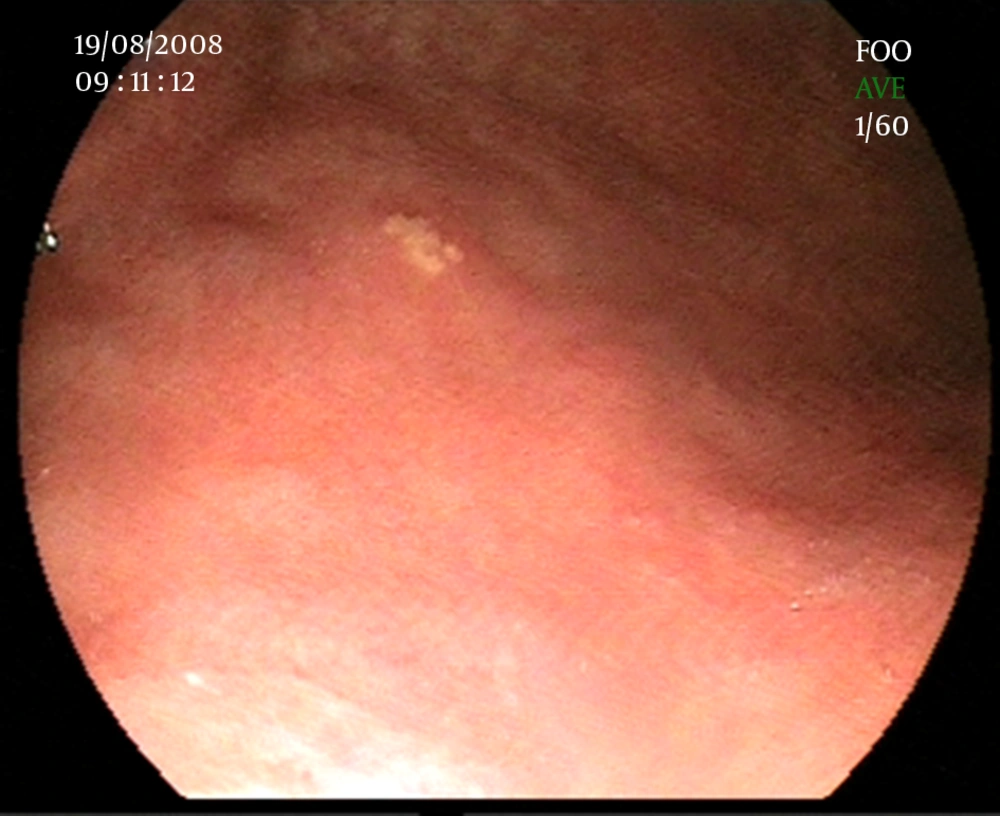
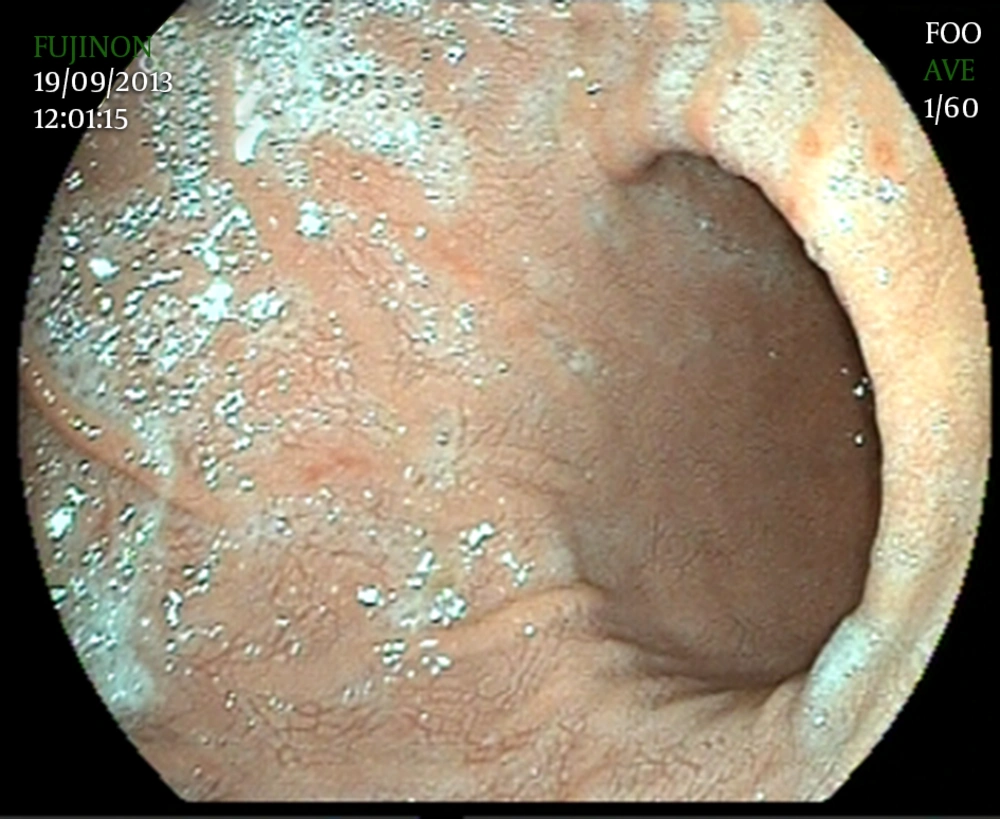
Since GXs are asymptomatic, they are diagnosed incidentally in endoscopic evaluation. They are commonly found in the antrum. Furthermore, GX has a typical endoscopic appearance of yellow-white, well-demarcated single or multiple nodules or plaques, with a size varying from 1 to 10 mm in diameter (2, 28) (Figure 2). However, unusual cases with rare findings of GX have been reported. We showed a diffuse gastric xanthomatosis, which had no plague formation in a 25 year-old female (Figure 3).
3.4.2. Histopathologically
GXs, like other xanthelasmas, are composed of large foamy cells containing a mixture of lipids, including cholesterol, neutral fat, low-density lipoprotein and oxidized low-density lipoprotein (29). These foamy cells are mostly histiocytes. However, plasma cells, smooth muscle cells, and Schwann cells may be involved when looking at the whole picture. Immunohistochemical studies should be used for differential diagnosis. The foamy cells in xanthomas usually display the marker CD68, a heavily glycosylated, 110-kDa membrane protein, which can be highlighted with monoclonal antibodies KP1 or PGM1, yet has a weak cytoplasmic positivity with periodic acid schiff (PAS) staining (30).
3.5. Differential Diagnosis
Some GI lesions show similar features as that of GX, endoscopically or histopathologically. However, clinical significance of those lesions is different from GX. Differential diagnosis should be made carefully.
Gastric fibrous xanthoma is a lesion that can reach giant sizes and cause significant GI bleeding. It is seen as a submucosal yellowish lesion in GI endoscopy and contains foamy cells, histologically (30).
Russell body gastritis (RBG) is a very rare disease where the lamina propria of gastric mucosa is excessively infiltrated by plasma cells containing Russell bodies, which are eosinophilic intra-cytoplasmic inclusions. Endoscopic images of RBG show whitish elevated lesions and can be mistaken as xanthoma, signet ring cell carcinoma, or malignant lymphoma (31).
Xanthogranuloma is a tumor that is macroscopically characterized by the formation of multiple golden yellow or bright yellow nodules, and histologically, the lesion is predominantly composed of foamy histiocytes mixed with acute and chronic inflammatory cells (32).
Pseudoxanthoma elasticum (PXE) is a hereditary connective tissue disorder characterized by disintegration and calcification of elastic fibers. Abnormal elastic fibers in the skin, retina and cardiovascular system produce characteristic manifestations in these areas. Patients with PXE may have linear or nodular, raised, submucosal lesions, which are yellow in color and similar to the xanthoma-like lesions. These lesions have high incidence of GI bleeding because of defects in the vascular component (33).
Signet-cell gastric adenocarcinoma is another differential diagnosis of the GX. Standard histology of xanthomas usually shows regular nuclear cells centrally located in the foamy cells, though atypical cells can be seen in cytology preparations. Masson trichrome staining can be positive in both entities. Periodic Acid Schiff staining is uniformly negative in GX and strongly positive in gastric signet-cell adenocarcinoma (34).
Gastric Xanthoma must not be confused with accumulation of lipid, submucosal lipoma, pseudolipomatosis, or accumulation of histiocytes without a visible lesion (35).
3.6. Treatment
There are no recommendations for the treatment of GX. However, in the literature some therapeutic studies have been reported. It has been proposed that proton pump inhibitors can decrease the intra-lysosomal acidity through inhibition of the lysosomal membrane and H+/K+ATPase. They prevent lipid destruction with this feature. Proton pump inhibitors also contribute to acceleration of mucosal healing and to treatment of H. pylori infection. These agents could afford protection against xanthoma formation (36).
3.7. Prognosis
Gastric xanthoma is known as a benign condition. However, there are no follow-up studies in the literature. It is unclear whether all detected lesions should be removed completely or followed up routinely. Because of their possible association with other potentially serious conditions of the stomach, the remainder of the gastric mucosa should be examined carefully and biopsy should be taken from the lesion during the upper GI endoscopy for diagnosis and ruling out gastric malignity.
4. Conclusions
We assessed the clinical impact of GX. For many decades GX has been reported as a benign condition. Because it has no clinical symptoms and signs, it is incidentally encountered during upper GI endoscopy.
Various pathogenic mechanisms have been suggested to explain the presence of xanthoma cells in GX. The latest concept in its etiopathogenesis is a healing response to local trauma or inflammation. This hypothesis is explained by the presence of GX accompanied with conditions, which cause gastric injury. However, the detailed developmental mechanism remains unknown. The histopathological findings are a diagnostic tool for these lesions. The presence of foamy histiocytes in the lamina propria is the main criterion for diagnosis. Immunohistochemical studies are used for differential diagnosis (3).
Clinical significance of GX is still unknown. However, there is increasing evidence on the association between GX and gastric injury. Because gastric injury leads to carcinogenesis, it may be a sign of chronic injury. Thus, it should not be ignored and concomitant conditions should be treated.