1. Background
Multiple sclerosis (MS), a serious demyelinating disease, is known to damage the central nervous system (CNS). This disease is recognized as the most important cause of non-traumatic neurological disorders in young adult populations, and its prevalence varies in different countries such as Iran (1-4). The histological analysis of MS indicates CNS regions without myelin and oligodendrocytes (5-7). These regions are developed due to the immunological destruction of myelin basic proteins (MBPs) (6). Similarly, traumatic brain injuries can lead to oligodendrocyte apoptosis and subsequent axonal demyelination, which can compromise nerve fiber integrity and function (8).
In mature and developing CNS, oligodendrocyte progenitor cells (OPCs) constitute a major population of glial cells (9, 10). These cells increase the level of myelinating oligodendrocytes in the CNS and are actively involved in CNS signaling (11). Therefore, introduction of exogenous in-vitro-cultured OPCs into chronically demyelinated lesions or induction of OPC differentiation from the pool of endogenous neural stem cells (NSCs) may be the viable strategies to promote remyelination and gain functionality (12-15).
Early OPCs are produced during oligogenesis in the ventricular zone, which migrate extensively to populate all CNS regions (16). OPCs first express olig2, which is a basic helix-loop-helix transcription factor. Neural progenitor cells (NPCs) differentiate into the oligodendroglial lineage through Olig2 (17-20). The oligodendrocyte lineage cells express different transcription factors selectively at different stages (19). Transcription factor, Sox10, contributes to oligodendrocyte fate regulation (21). In Sox10-deficient transgenic mice, oligodendrocyte progenitors cannot terminally differentiate into mature myelinating oligodendrocytes, as Sox10 regulates myelin gene expression and results in oligodendrocyte terminal differentiation (21-24).
Recent studies on herbal medicines are shown that the extracts of saffron, a famous Persian food additive, can have many therapeutic properties, such as neuroprotective effects on neural injuries and enhancing cognitive functions (25-27), as well as anticancer, antidepressant, anti-atherosclerosis, and anti-inflammatory activities (28-32). The major active component of saffron, responsible for its red color, is crocin, which is a water-soluble carotenoid with antioxidant activities. It constitutes 6% - 16% of saffron dry mass and includes a group of crocetin glycosides (32, 33).
In an experimental autoimmune encephalomyelitis (EAE) model, crocin could prevent demyelination and neurodegenerative activities (34). In addition, it has anti-inflammatory effects on brain microglial cells (34, 35).
2. Objectives
Despite the neuroprotective effect of crocin, its effects on oligodendrogenesis and maturation are unclear. Therefore, the current study aimed at evaluating the effect of aquatic saffron extract and crocin on the differentiation of NSCs into oligodendrocytes by evaluating olig2 expression, as an early marker of OPC, and sox10, as an essential factor for OPC maturation.
3. Methods
3.1. NSC Isolation
NSCs were extracted from the cortex of E14 rat embryos (36). After harvesting the embryos under sterile conditions and washing them 3 times with cold PBS (phosphate buffered saline), supplemented with 10% penicillin/streptomycin, the cortices extracted from several embryos were microdissected under a stereo microscope and pooled. By adding 1 mL of NSC medium and pipetting up and down gently in a 15-mL falcon tube, the cortical tissue was dissociated into a single-cell suspension mechanically (36).
After centrifugation for 5 minutes at 110 g, cells were resuspended in the NSC medium, containing Gibco™ Dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12) (GIBCO, USA), supplemented with 1% N2 and 2% B-27 (GIBCO, Carsbald, CA, USA). The cells were then added to a plate at 2 × 105 cell/mL in 5 mL medium, supplemented with basic fibroblast growth factor (10 ng/mL of bFGF; Sigma-Aldrich, USA) and 1% penicillin/streptomycin. Afterwards, the cells were incubated for 5 days in a humid chamber (5% CO2) at 37°C to form neurospheres.
3.2. Alamar Blue® Assay
For the Alamar Blue® assay, 96-well plates (Nunc, Denmark) were covered with poly-D-lysine-coated (200 µg/mL) coverslips. To determine the optimal cell density, serial concentrations of cells (5 - 25 × 103 cell/mL) were added to the coated plate wells. Then, 10% Alamar Blue® (Sigma-Aldrich, MO, USA) was added to each well, and absorbance was read at 590 nm after 90 minutes. Subsequently, cells with optimal density were plated in wells and treated for 2 and 5 days with different concentrations of crocin (Sigma-Aldrich, MO, USA; 1.6, 3.12, 6.25, 12.5, 25, 50, 75, 100, 150, 200, 300, and 400 µM) and saffron (Estahban, Fars Convince, Iran; 0.65, 1.25, 2.5, 5, 10, 20, 40, 80, 160, 320, and 640 mg/mL). The process of absorbance reading was repeated, and effective concentrations (EC50) of saffron and crocin were calculated based on the IC50 curve.
3.3. Treatment with Crocin and Saffron
The cells (50 × 103) were seeded on a 96-well plate covered with poly-D-lysine (Sigma-Aldrich, MO, USA); then, they were treated to evaluate the differentiation of NSCs into OPCs. NSCs were cultured in complete NSC medium under 4 different treatment conditions: 1, The negative control, 10 ng/mL of bFGF; 2, The positive control, 10 ng/mL of bFGF plus 30 ng/mL of platelet-derived growth factor-AA (PDGF-AA; Sigma-Aldrich, MO, USA); 3, The Crocin group, crocin extract plus 10 ng/mL of bFGF; and 4, The Saffron group, saffron extract plus 10 ng/mL of bFGF. Cell treatment was carried out for 2 or 5 days under the certain conditions.
3.4. Flow Cytometry
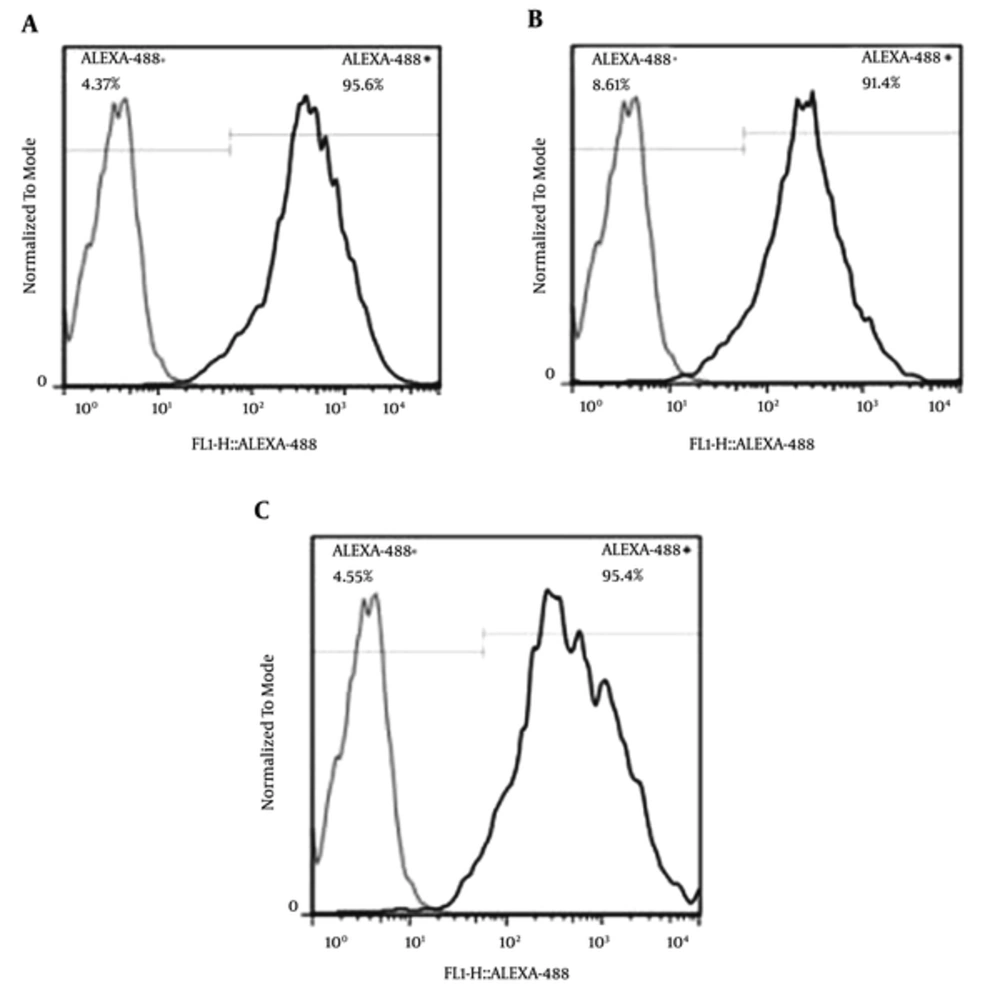
Two days after treatment, the cells were detached with trypsin-EDTA (ethylenediaminetetraacetic acid) (GIBCO, Grand Island, NY, USA). The enzyme activity was also quenched by adding soybean trypsin inhibitor in an equal volume (Sigma-Aldrich, MO, USA). It was then prepared for flow cytometry, as described in the literature (37). Briefly, cell centrifugation was performed, and 2% paraformaldehyde was used to fix the cells in 10 minutes. Then, they were washed 3 times and incubated with diluted rabbit monoclonal IgG1 anti-Olig2 (Abcam, UK, 1:250) at room temperature for 45 minutes.
Afterwards, 488 goat anti-rabbit IgG (Alexa Fluor®, Eugene, OR, USA, 1:1000) was added, and the samples were incubated in darkness for 45 minutes. The cells were then washed 3 times and suspended in 400 µL PBS. Finally, flow cytometry and data analysis were performed using a FACSCalibur™ system and FlowJo 7.6 software, respectively. At this stage, olig2 expression was assessed after 1, 2, and 5 days; the optimal time for olig2 expression was 2 days.
3.5. RNA Extraction and Evaluation of sox10 mRNA Expression by RT-PCR method
Total RNA isolation was performed in different groups of treated cells, using Biozol reagent (Bioflux, Japan), and total RNA was used to synthesize cDNA (1 µg). Real time-PCR was performed in a 20-μL reaction volume system (Fermentas, Burlington, Canada). The 25-μL PCR reaction system included 2 µL cDNA, 1 µL primers (Table 1), 0.5 µL dNTP mixture (2.5 mM each), 10X PCR buffer (2.5 µL), 1.5 µL MgCl2, and 0.3 µL Taq DNA polymerase (5 U/µL).
| Primer | Forward | Reverse | Ta |
|---|---|---|---|
| sox10 | 5’-CTGAACGAGACAAG-3’ | 5’-AACAACCTCTTCGTCCGTAC-3’ | 58 |
| GAPDH | 3’-CTTGTCTCGTTCAG-5’ | 3’-CGAAGGTGGAAGAGTGAGT-5’ | 58 |
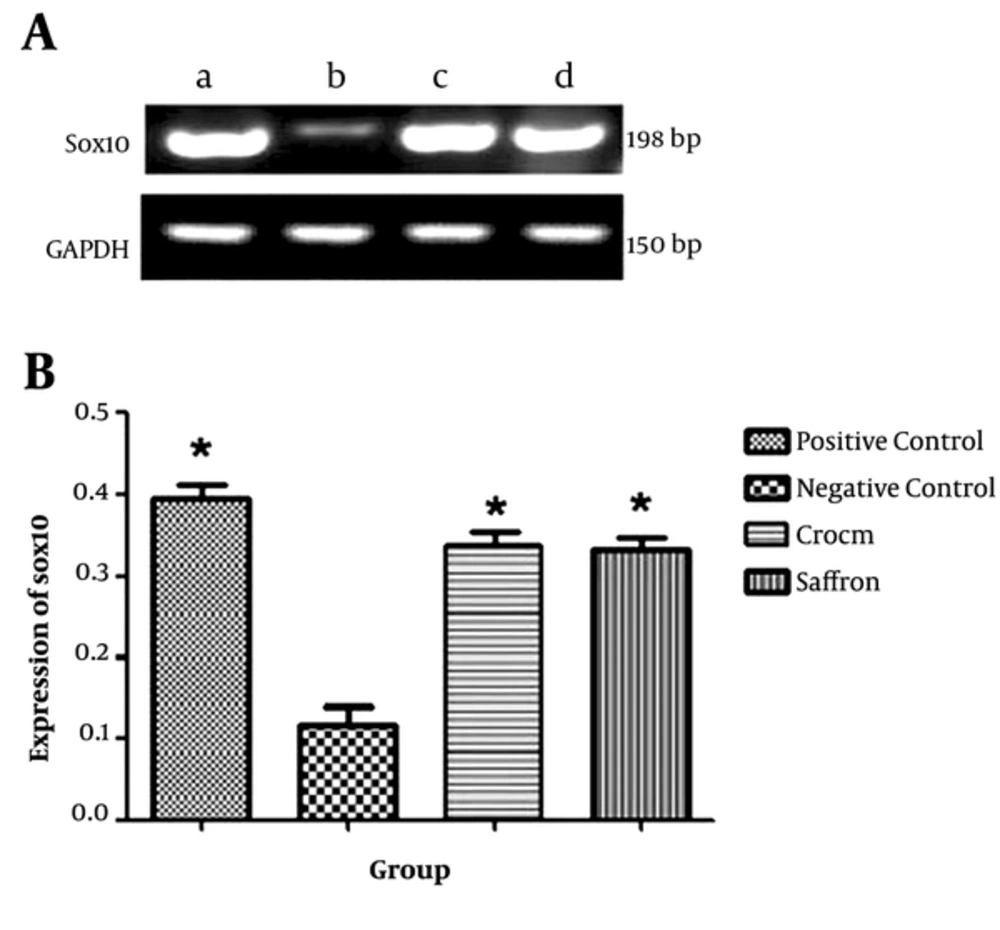
The PCR conditions were as follows: 5 minutes at 95°C, followed by 30 cycles at 94°C for 30 seconds, 58°C for 45 seconds, 72°C for 1 minute, and 72°C for 10 minutes. Then, on 2% agarose gel, the products were separated, and visualization was performed using GelRed staining. Accordingly, a 198-bp amplicon was expected from PCR final product for sox10. Appropriate primers for the analysis of GAPDH mRNA as an internal control as well as sox10 gene, are demonstrated in Table 1. The internal control primers should also produce 150-bp amplicons(Figure 3).
3.6. Quantitative Real Time-PCR
A real-time PCR system (ABI, PCR 7500) was used to perform the assay. The reaction mixture (total volume of 20 µL) consisted of 2 µL cDNA (diluted 10 times), 0.5 µL of primer solutions (5 mM/L), and 10 µL SYBR green DNA PCR Master Mix. For real-time PCR amplification, precycling heat activation was performed on DNA polymerase for 2 minutes at 50°C, followed by 30 cycles of denaturation at 95°C for 15 seconds, and annealing at 58°C for 1 minute.
For melting curve analysis, 70 cycles were run with temperature increase (rate, 0.5°C/cycle; initial temperature, 60°C) for 10 seconds. The amplification efficiency was examined relative to the standard curve slope (r2 > 0.9888). The relative expression level (fold changes) of sox10 gene was calculated by 2-ΔΔCT formula. GAPDH gene was also considered as the internal control.
3.7. Statistical Analysis
Data were analyzed with GraphPad Prism software version 6.01 (San Diego, CA, USA) using one-way ANOVA followed by Tukey post-hoc test. P < 0.05 was considered as the level of significance.
4. Results
4.1. Determination of the Optimum Non-Toxic Concentrations of Saffron and Crocin on NSCs
By the use of Alamar Blue® cell viability assay and assessment of different concentrations, 20 µg/µL versus 2.5 µg/µL saffron and 25 µM versus 12.5 µM crocin were the optimum non-toxic concentrations for 2- and 5-day treatment courses, respectively.
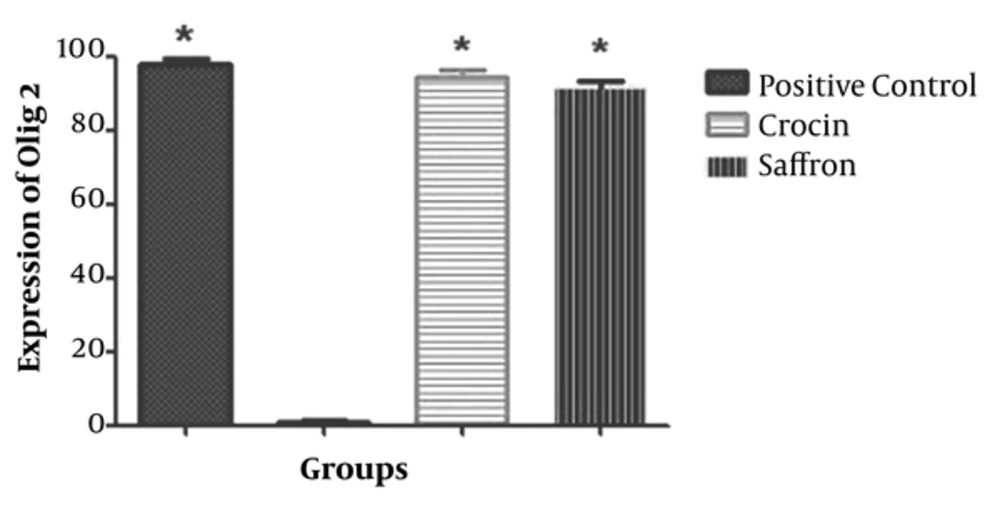
4.2. Saffron and Crocin Effects on the Expression of olig2
As shown in Figures 1 and 2, the level of Olig2 protein was significantly different between the negative control group and those treated with saffron and crocin (P < 0.05); the latter groups results were similar to that of the positive controls (Figures 1 and 2).
4.3. Effects of Saffron and Crocin on sox10 mRNA Expression Based on Real-Time PCR
The level of sox10 mRNA increased during the treatment with crocin and saffron extract (P < 0.05) (Figure 3). Moreover, as shown in Tables 2 and 3, the mean expression level of sox10 gene in the positive control, saffron, and crocin groups was 1 ± 0.012, 0.72 ± 0.13, and 0.835 ± 0.015, respectively; the difference among the groups were statistically insignificant (P > 0.05).
A, sox10 and GAPDH levels were evaluated by real-time PCR in different groups, a, positive control; b, negative control; c, saffron, and d, crocin. B, Results of mRNA expression in different groups by real-time PCR; according to diagram, there is no significant difference between the positive control, and saffron and crocin groups (*P > 0.05). Each bar represents the mean ± SEM.
| Different Groups | Sox10 mRNA Expression (Mean) | Standard Deviation |
|---|---|---|
| Negative control | 0.27 | 0.015 |
| Positive control | 1 | 0.012 |
| Crocin | 0.835 | 0.015 |
| Saffron | 0.72 | 0.13 |
| Tukey Multiple Comparison Test | P Value |
|---|---|
| Negative control vs. positive control | P < 0.05 |
| Negative control vs. crocin | P < 0.05 |
| Negative control vs. saffron | P < 0.05 |
| Positive control vs. crocin | P > 0.05 |
| Positive control vs. saffron | P > 0.05 |
| Crocin vs. saffron | P > 0.05 |
5. Discussion
In the current study, the impact of saffron and crocin extracts on NSC differentiation into OPCs was investigated. Treatment of NSCs with the studied agents significantly increased OPC differentiation, as evidenced by the high expression of early and late OPC markers, olig2 and sox10, respectively (P < 0.05). No significant difference was observed among the positive control, saffron, and crocin groups in terms of sox10 mRNA and olig2 expression. According to the findings, crocin and saffron may induce differentiation of NSCs into OPCs. To the best of authors’ knowledge, no study reported the OPC-inducing effects of saffron and crocin on NSCs differentiation.
Baharara et al., indicated that saffron extract, if used in combination with vitamin D3, can have synergistic effects on osteogenic mesenchymal stem cell differentiation in the bone marrow of rats (38). Crocin also acts as a potent antioxidant, which decreases neuronal cell death induced by ischemic stress through increasing glutathione level and inhibiting the c-Jun N-terminal kinase (JNK) pathway (25). Furthermore, safranal, crocin, and crocetin show neuroprotective effects by decreasing the neurotoxic molecular level in activated microglia (35), as well as hippocampal tissue (39).
It is shown that crocin and crocetin can inhibit nitric oxide release from microglia triggered by interferon-gamma and amyloid-beta (35). In addition, inflammatory gene expression, endoplasmic reticulum stress in the spinal cord, and neurobehavioral deficits were ameliorated by crocin treatment in an experimental EAE model (34). Regarding the anti-inflammatory effect of crocin, Wang et al., reported that pretreatment with crocin resulted in neuroprotective effects on traumatic brain damage, and decreased microglial activation, pro-inflammatory cytokine release, and apoptosis by activating Notch signaling pathway (40). The present results demonstrated that crocin and aquatic extract of saffron could increase the level of Olig2 as an appropriate marker for oligodendrocyte differentiation. These differentiated cells are also potentiated to turn into mature OPCs due to increased sox10 mRNA level in the treated group. According to some evidence, sox10 is among olig2 downstream targets (22-24). It is shown that noggin, as an antagonist of bone morphogenetic proteins (BMPs), induces the expression of sox10 in the differentiation of retinoic acid-treated human embryonic stem (HUES) cells (41). Literature showed that the endogenous BMP-2 signaling pathway is under the control of the commitment stage in embryonic stem cell differentiation into different lineages (42). Moreover, to produce mature oligodendrocytes from HUES cells, Olig1/2, Sox10, and Nkx2.2, are essential (41).
According to the results of a study, BMPs are triggered by retinoic acids in HUES cells, and noggin is necessary in the formation of mature oligodendrocytes producing MBPs. Pretreatment of such precursor cells with noggin stimulates the production of brain MBP+ fibers in mice with MBP deficiency (41). In another study, Lu et al., showed that treatment with noggin-modified bone marrow stromal cells and/or brain-derived neurotrophic factor could inhibit inflammation and apoptosis induced by ischemia in an ischemic stroke animal model. This might result from the upregulation of BCL-2 and p-GSK3β/p-Akt pathways, besides Bax downregulation (43).
The mentioned findings indicated the increase of sox10 expression without noggin treatment. Therefore, it can be concluded that pretreatment with crocin and saffron extracts could compensate for the noggin effect in the differentiation process. Finally, culture supplementation with crocin or saffron at certain stages (e g, incubation) might be involved in MBP+ oligodendrocyte generation in NSCs.
The present findings indicated the potential application of saffron in the inhibition of inflammation pathways, as well as treatment of neurodegenerative disorders such as MS. However, further research is required to clarify the effect of OPC pretreatment with crocin and saffron as well as the role of BMP proteins and noggin signaling before transplantation. In addition, their myelination potential in different brain regions should be evaluated in future studies. The present findings should also be analyzed in MBP-deficient mice to examine and compare the myelination potential of donor cells.
5.1. Conclusion
Treatment of NSCs with saffron and crocin significantly increased OPC differentiation, as evidenced by the high expression of early and late OPC markers, olig2 and sox10, respectively. These 2 agents could improve human-modified NSC-based therapy by considering their medical applications for remyelination or neuroprotection purposes.