1. Background
It is known that ultraviolet (UV) radiation has a potent effect on biological organism (1). Many studies have shown that UV radiation leads to immune suppression, DNA damage, apoptosis, skin carcinogenesis, oxidative damage, tumor formation, and inflammation (2, 3). UV radiation is typically classified into 3 groups based on wavelength, including UVA (320 - 400 nm), UVB (290 - 320 nm), and UVC (200 - 290 nm) (4). One of the most significant sources of indoor UV radiation are compact fluorescent lamps (CFLs). The CFLs emit UV radiation (5). In 1985, Cole et al. (5) demonstrated that CFLs emit UVB and UVC. Recently, Sayre et al. (1) demonstrated that CFLs emit UVA, UVB, and even UVC. Some studies have demonstrated that UVB radiation causes oxidative damage to the skin, resulting in photo aging and skin cancer (5-7). UVB radiation can be effected directly by inducing inflammation and proliferation in human and animal skin as well as damaging eye cells, or indirectly by producing reactive oxygen species (8, 9). The most mutagenic and carcinogenic properties of sunlight can be attributed to chronic exposure to UVB radiation (10).
Recent study demonstrated that acute exposure to UVB and UVA radiation changes in oxidative stress-related biomarkers in the liver and blood of hairless mice (11). Plasma adiponectin level has been reduced after UVB radiation by affecting visceral adipose tissues (12). Our previous study showed that UVB and UVA emitted by CFLs increased the level of serum ALT, AST, and cholesterol, as well as appearance changes of liver in wistar rats (not published). The liver is the largest solid organ of the body and plays a crucial role in humans and animals lives due to the fact that it performs numerous essential tasks including: regulates the levels of most chemicals in the blood, excretes bile, detoxifies harmful compounds, and supports the function of other organs in the body, thus helping homeostasis (13-15).
Curcumin, a bioactive component, has been derived from the rhizome of turmeric (16). In recent years, many studies have suggested that curcumin has wide-spectrum activity such as anti-oxidant properties, anti-inflammatory, anti-cancer, anti-myeloid, anti-arthritis, and antimicrobial activities (17-20).
The focus of this study is to assess the effect of curcumin as a protective factor in complications of CFLs on liver function.
2. Methods
Study design: experiments were carried out on 24 adult male wistar rats (230 ± 30 g body weight). The animals were randomly divided into 3 groups: group 1: control (received ethyl oleate 0.2 mL, IP, for 40 days, without CFLs exposure), group 2: fluorescent (received ethyl oleate 0.2 mL, IP, daily and treated with 12 hours CFLs exposure for 40 days, and Group 3: curcumin (Pretreatment with Curcumin (20 µ mol, IP) along with 12 hours CFLs exposure for 40 days).
All the procedures were performed according to the “Principles of Laboratory Animal Care” (NIH publication No. 85-23, revised 1985), as well as the specific rules provided by the animal care and use committee of national medical and health service.
A total of 4 commercial CFLs (40 w) were purchased for this study. Following a 10 min warm up period, the UV emission from each lamp was measured by UV meter model UV-340 A. The animals were placed in boxes covered with aluminum sheets. The size of each box was one square meter. We used 4 boxes in this experiment (2 boxes with 2 lamps in each and 2 boxes without any lamps).
The CFLs were put at a distance of 10cm from rats emitting essentially at 280 - 400 nm and divided into 2 distinct spectral areas including UVA and UVB. The intensity of each lamp was UVA (1.06 W/m2) and UVB (0.02 Wm2).
A total of 24 hours after UV exposure, the animals were anesthetized by pentobarbital (40 mg/kg, IP). After deep anesthesia, blood samples were collected from the right atrial of rats into glass tubes without any anticoagulants. After clotting for 20 minutes, the blood samples were centrifuged in 3000 g for 10 minutes at 4o by laboratory centrifuge (co.ltd, Roto-uni Germany). Next, serums were removed from the samples, transferred to plastic vials, stored in a refrigerator, and then frozen at -80oC until examination.
Enzymatic and biochemical analyses: The activity of enzymes (ALT, AST, AMY, ALP) and biochemical levels of serums (Chol, TG, low-density lipoprotein (LDL), TB, DB) were measured using an auto-biochemical analyzer (BT 3000).
Histological study: after blood sampling and incision in the abdomen, the liver was removed. The livers were then fixed in a neutral-buffered 10% formalin solution for hematoxylin and eosin staining (H&E). Next, they were processed routinely, embedded in paraffin, sectioned 5 µm thicknesses, and stained with H&E. At the end of the procedure, the samples were examined by light microscopy. Morphological analysis was performed by using a computerized image analysis system on 10 and 40 microscopic fields per section and the examination was carried out at 100- and 400-fold magnification (CIA-102; Olympus).
Statistical analysis: Data were expressed as the mean ± SD. Statistical significance of differences was assessed with one-way ANOVA followed by Tukeys’ test. P value less than 0.05 was considered as statistically significant.
3. Results
Finding of biochemical and morphology data in the control, fluorescent, and curcumin groups at 40 days after CFLs exposure and administration with curcumin are shown in Table 1 and Figures 1 and 2.
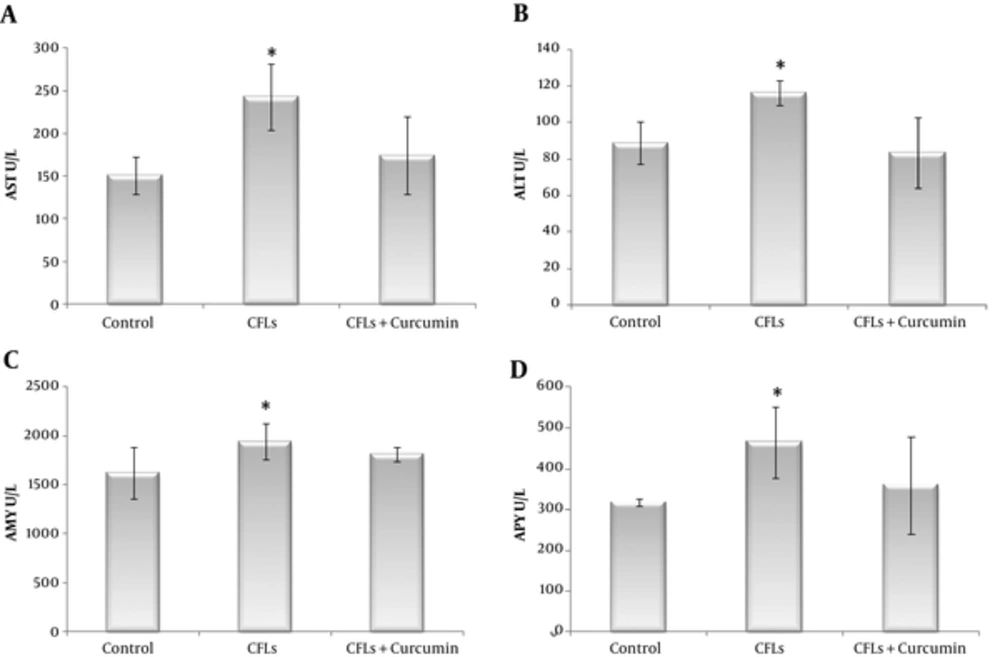
As it is shown in Table 1, the findings of this study indicated that CFLs increased some biochemical data in rats after exposure to CFLs for 40 days. Serum AST, ALT, Chol, TG, LDL, TB (Total Bilirubin, DB (Direct Bilirubin), AMY, and ALP levels were increased in the Fluorescent group compared with the control group (Table 1 and Figure 1).
In the fluorescent group, the level of AST was significantly increased compared with the control group (243.25 ± 38.85 U/L and 151.25 ± 21.64 U/L, respectively, PV < 0.0001). When we used curcumin following CFLs, the levels of AST was reached to the normal group (174.25 ± 45.47 U/L, PV = 0.437). Therefore there were no significant differences at the control and curcumin groups (Figure 1A).
In the fluorescent group, the level of ALT was significantly increased compared with the control group (116.32 ± 6.89 U/L and 88.75 ± 11.6 U/L, respectively, PV= 0.002). When we used curcumin following CFLs, the levels of ALT was decreased to the normal group (83.57 ± 19.25 U/L, PV = 0.729). However there were no significant differences between the control and curcumin groups (Figure 1B).
The TG level in the fluorescent group was no significant difference in comparison with the control group (89.83 ± 14.13 mg/dL and 78.25 ± 9.67 mg/dL, respectively, PV = 0.103). Curcumin administration following CFLs, significantly decreased the levels of TG as compared with the control group (62.28 ± 7.26 mg/dL, PV = 0.019) (Table 1).
The cholesterol level in the fluorescent and curcumin groups had no significant difference with control group, respectively (83.86 ± 6.66 mg/dL, 71.63 ± 14.12 mg/dL, and 73 ± 9.47 mg/dL, respectively PV = 0.124, PV = 0.963) (Table 1).
LDL was significantly higher in the fluorescent group than the control group (18.9 ± 1.97 mg/dL and 13.50 ± 1.57 mg/dL, respectively, PV = 0.001). Even after administration of curcumin, it was slightly more than the control group. However, this difference was not significant (15.78 ± 3.45 mg/dl, PV = 0.181) (Table 1).
The TB (Total Bilirubin) and DB (Direct Bilirubin) levels were significantly increased in the fluorescent group compared with the control group, respectively (0.48 ± 0.06 mg/dL, 0.26 ± 0.13 mg/dL, 0.24 ± 0.02 mg/dL, and 0.11 ± 0.01 mg/dL, respectively, PV < 0.0001, PV < 0.0001). After administration of curcumin following CFLs, the levels of BT and DB were reversed to the normal group (0.26 ± 0.07 mg/dL, 0.13 ± 0.01 mg/dL, respectively, PV = 0.787, PV = 0.551) (Table 1).
As shown in Table 1, AMY level was significantly higher in the fluorescent group than in the control group (1942.4 ± 180.77 U/L and 1623.3 ± 267.06 U/L, respectively, PV = 0.009). When we used curcumin following CFLs, there was no significant difference between curcumin and the control group (1811.6 ± 78.41U/L and 1623.3 ± 267.06 U/L, respectively, PV = 0.145) (Figure 1C).
The level of ALP was significantly increased in the fluorescent group compared with that in the control group (465.38 ± 87.9 U/L and 317.5 ± 9.24 U/L, respectively, PV = 0.006). Whereas, there was no significant difference between in the curcumin and control group (360.25 ± 118.64 U/L and 317.5 ± 9.24 U/L, respectively, PV = 0.584) (Figure 1D).
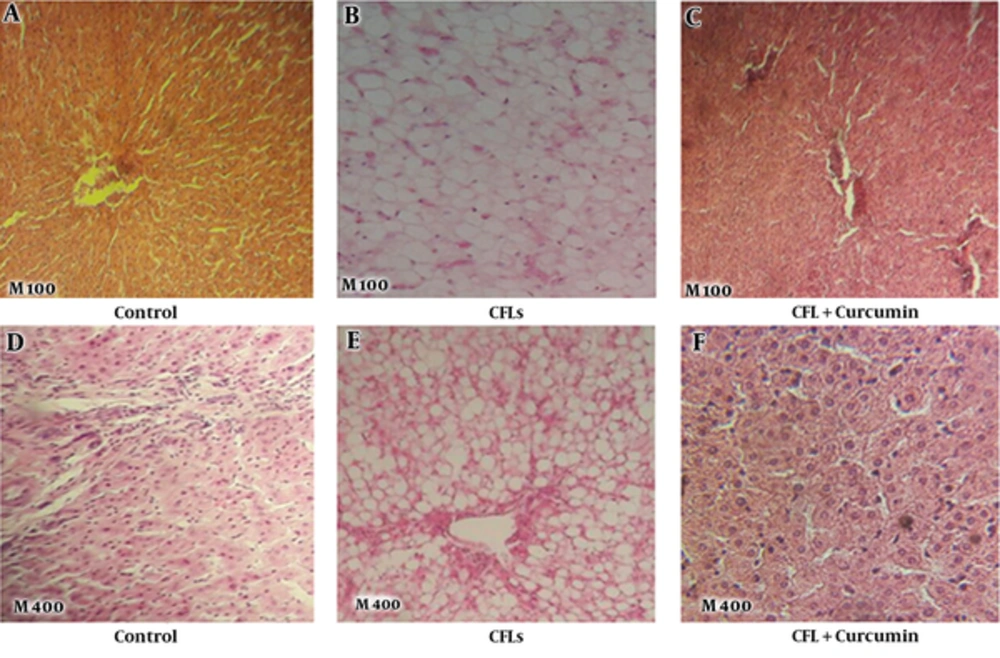
As it is shown in Figure 2, histological of the rat livers with staining H&E in the control group, without CFLs exposure and without administration of curcumin indicated that the livers of rats were normal section. There was no lipid accumulation on the livers of rats. The liver tissues, hepatic cells, and portal veins were normal (Figure 2A and 2D). When the CFLs were used, the accumulation of fat inside the hepatic cells was significantly observed. Moreover, the cells were inflamed (Figure 2B and 2E), however, curcumin prevented this changes (Figure 2C and 2F).
4. Discussion
The results of the present study showed that the CFLs cause a significant increase in liver enzymes including AST, ALT, ALP, and also AMY. The first 3 enzymes respectively are commonly increased in liver disorders (21). Indeed, serum AST, ALT, and ALP levels were released by damaged hepatocyte cells into blood circulation (22). Serum amylase elevation is a reflective of pancreatitis (23). This elevation may be effected by UV radiation. Because the CFLs, emits ultraviolet radiation spatially UVA, UVB and even UVC (1, 4). Many studies have shown that UV radiation can cause DNA damage, cellular aging, oxidative damage, production of free radicals, and tumor formation (2, 15). A recent study reported that UV radiation changes oxidative stress- related biomarker in the liver (11) due to the fact that the oxidative stress is due to over production of reactive oxygen species that can induce oxidative damage to vital cellular molecules and structures (24). Therefore, the results of this study are similar to the above mentioned studies.
In this study, we found that curcumin prevented of increase liver enzymes induced by CFLs. Curcumin is a main component of turmeric that has many biological and pharmacological activities (25, 26). This prevention of curcumin may be due to antioxidant and anti-inflammatory effects. Since, one of the most complications of UV radiation is oxidative damage (7). Many studies have shown that curcumin has antioxidant and anti-inflammatory activities (27).
This study showed that the levels of TG, chol, LDL, and bilirubin increased the following CFLs exposure. Plasma triglyceride is primarily produced by the intestine and liver. Hypertriglyceridemia can be related by deficiency of high density lipoprotein (HDL), due to the fact that liver damage produces low amount of HDL (28). This damage of liver can be due to UV radiation (11). Although hypercholesterolemia is produced in our experiment with liver damage, in order that the synthesis of cholesterol occurs in many cells for example intestine, adrenal cortex and reproductive tissues (29). Recently, studies showed that high intensity and prolonged fluorescent exposure lights are harmful to all living organisms and produce hemolysis of erythrocyte, this results in an increase in plasma bilirubin (30, 31). Therefore, our results confirmed previous studies. When we used curcumin, the levels of cholesterol, LDL, and bilirubin have reached the normal levels, however, the level of TG was significantly decreased.
In this study, the liver of CFLs rats displayed a significant increase in the intracellular lipid of hepatic cells. Accumulation of fat in the hepatic cell is 1 of the complications of exposure to CFLs. Results of our pervious study showed that the CFLs produced fatty liver disease (FLD) (not published). FLD is defined as the accumulation of lipids in the hepatocytes’ cytoplasm, which is usually accompanied with an increase in liver volume (32) and elevation of liver enzymes such as ALT, AST, and also increase TG and TB serum with visceral adiposity (33, 34). Many factors are involved in causing the FLD, including alcohol consumption, nutritional disorders, dyslipidemia, insulin resistance, obesity, hypertension, infectious diseases, hepatitis C virus, and genetic factors (35-38). The results of the current study showed that administration of curcumin prevent liver damage and accumulation of lipid in hepatic cells induced by CFLs.
Conclusion: In summary, in this study we found out that the CFLs increased liver enzymes, produced FLD and damaged the liver tissue. These disorders may be the effects of UV radiation emitted by CFLs; however, curcumin, as an antioxidant, has a protective effect on disorders such as biochemical and morphological changes evoked by CFLs of rat liver.