1. Background
Diabetes mellitus is a serious metabolic disorder with functional and structural impacts (1). Oxidative stress has a fundamental role in the progression of diabetes complications (2). Within and following diabetes, non-absorbed glucose in fat tissue and muscles leads to the elevation of glucose concentration in the blood. The excessive glucose increases the oxidant product and devastates the antioxidant defense system (3).
Reduced CAT activity and increased MDA levels are considered as indicators of oxidative stress (4, 5). CAT is one of the effective enzymes known to protect cells from hydrogen peroxide generated within the cells (6) and MDA is a toxic product of polyunsaturated fatty acid peroxidation (7).
It is believed that diabetes has undesirable impacts on the function of the reproductive system in men like decreasing plasma testosterone levels and sperm count and making apoptosis in the testicular germ cells (8). Besides, diabetes in the testicular tissue of rats brings about hypo spermatogenesis changing in the dynamics of testicular microvascular blood flow, endocrine signaling, and male infertility by increasing lipid peroxidation and ROS production (9).
The testicular tissue because of several reasons is extremely predisposed to the activity of oxidative stress and free radicals due to containing high levels of unsaturated fatty acids, high rate of cell division, high rate of cell competition for oxygen, and low oxygen pressure due to weakened vessels. Additionally, since the body’s antioxidant system is not capable of invalidating all free radicals, antioxidant supplements are suggested to reduce the adverse impacts of oxidative stress, elevate spermatogenesis, and enhance fertility (10).
Synthetic antioxidants are often associated with undesirable side effects and high cost for patients; hence, herbal plants are alternative therapies because of anti-oxidant properties, low-cost treatment, reliability, and fewer side effects. Stevia rebaudiana Bertoni as an herbal medicine is a sweet and nutrient-rich plant, and it is effective in the regulation of blood glucose. The sweet taste of Stevia leaf is due to the presence of steviol glycosides in its leaves (10). Stevia and its glycosides have therapeutic effects against several diseases such as cancer, diabetes mellitus, inflammation, obesity, and tooth decay (11-15).
Since the control of carbohydrate intake and compliance with a low-calorie diet are important for successful remedy of diabetes, consuming Stevia as an impressive plant in diabetes treatment is affordable (16). Clinical trial studies have proven that the leaf extract of Stevia rebaudiana decreases blood glucose in diabetic patients (16, 17). Another study showed that stevioside prevents the absorption of glucose in the duodenum of rats (18).
It has been well documented that the aquatic extract of stevia decreases the serum level of Interleukin-6 as an inflammatory factor, in STZ-NA-induced diabetic rats (19). Moreover, a recent study has shown that stevia by increasing the insulin level exerts profitable anti-hyperglycemic effects via a PPAR-dependent mechanism and antioxidant activities in pancreatic tissues could be effective in diabetic rats (20).
Nowadays, it is believed that one of the adverse impacts of diabetes on the male reproductive system is increasing the MDA level and decreasing the catalase activity (9). Since stevia has an antioxidant nature and the effects of stevia on oxidative stress in the testicular tissue of diabetic rats are unclear, the current study focused on stevia for the treatment of diabetes by diminishing oxidative stress.
2. Methods
2.1. Chemicals
STZ (Streptozotocin), NA (Nicotinamide), TBA (thiobarbituric acid), and TEP (1, 1’, 3, 3’- tetra ethoxy propane) were provided by Sigma (Germany). In addition, metformin was supplied by Sigma (USA); Stevia leaves were purchased from Golsaran company, Rasht, Iran.
2.2. Animals
Adult male Wistar rats (4 months-old, 200 - 250 g) were procured from Shiraz University of Medical Science. All rats were acclimatized to new environmental conditions for one week, prior to initiation of the experiment. The rats were kept under standard laboratory conditions of light/dark cycle (12/12 hours), fed with standard diet, chow diet (Pars Dam Co, Tehran, Iran), and water ad libitum. The experimental rats were divided into 5 groups each containing 10 rats. The study was conducted in accordance with the principles of institutional animal ethics.
2.3. Experimental Design
Diabetes was induced chronically by an intraperitoneal injection of NA (Nicotinamide, 120 mg/kg b. wt., dissolved in normal saline) 15 minutes before the single-dose administration of STZ (Streptozotocin, 60 mg/kg b. wt., freshly prepared in citrate buffer, pH 4.5). Following the diabetes induction, blood was drawn from the tail vein of the experimental rats for determining their fasting blood glucose levels. Rats with fasting blood glucose levels of more than 250 mg/dL were considered diabetic and selected for the treatments using metformin (500 mg/kg) and aquatic extract of stevia (400 mg/kg). Within six weeks, our cohort comprised the following groups: 1. Group A: water, 1 mL (Control) and 2. Group B: water, 1 mL (diabetic control). Four weeks after the induction of diabetes, the diabetic rats were randomly divided into two groups: 3. Group C and 4. Group D: diabetic groups treated with the aquatic extract of stevia (400 mg/kg) and metformin (500 mg/kg), respectively; these groups received the treatment by gavage in a single dose every morning for two weeks; and 5. Group E: healthy group treated with the aquatic extract of stevia (400 mg/kg).
2.4. Preparation of Aquatic Extract of Stevia
Stevia leaves were purchased from Golsaran Company (Rasht, Iran) from March 10 to 29. The dried leaves were finely powdered with the help of a grinder. In the next step, the powder was extracted in a Soxhlet apparatus and evaporated to dryness under decreased pressure in a vacuum rotary evaporator. The extract was air-dried up to solid to obtain a semi-solid mass. Next, 100 g of the powder extract suspension was mixed in 1200 mL of distilled water and kept in dark for 24 hours. Subsequently, the solution was filtered and the extract was evaporated into a rotary evaporator at the temperature range of 40 - 50°C. To confirm that the extraction is dry and has no humidity, it was placed in a vacuum desiccator for 24 hours; hence, the efficiency was calculated (20).
2.5. Glucose Measurement
The serum glucose level was measured after six weeks of treatment by using prestige instrument (Hitachi, Japan) and diagnostic colorimetric kits (BioSystem, Spain).
2.6. Preparation of Homogenate from Testicular Tissue
Testes were separated, frozen, and rapidly homogenized in 5 mL cold-normal saline with the aid of homogenizer (Potter Elvehjem, Bodine Electric, Chicago, IL, USA). The homogenate was filtered and then centrifuged at 10,000 g for 1 hours at 4°C. The supernatant obtained was used for the estimation of total protein, MDA, and CAT activity.
2.7. Determination of Malondialdehyde (MDA)
The MDA level was measured using Hagar et al.’s method (21). In short, since aldehyde is not stable, MDA was supplied as an acetal derivative. Within the acid incubation step at 95°C, the acetal form (TEP or 1, 1’, 3, 3’-tetra ethoxy propane) was hydrolyzed to form MDA. The TEP standard was supplied as a 10 mM stock solution in Tris-HCl and diluted 1/500 (v/v) in water. Finally, MDA concentrations were detected according to a standard curve at 532 nm.
2.8. Determination of Catalase (CAT) Activity
Catalase (EC 1.11.1.6) was measured according to the Aebi’s procedure based on the content of H2O2 that decomposed (22). In short, 66.7 mmol/L phosphate buffer (pH = 7.0), 20 uL of pancreas supernatant, and 355 uL of distilled water were added to 75 uL of H2O2 (120 mmol/L) and the utilization of H2O2 was followed spectrophotometrically at 240 nm for 3 minutes at 25°C. The catalase activity was expressed as μmol H2O2 consumed/min per mg testis supernatant protein using a molar extinction coefficient of 43.6 L/mol per cm for H2O2.
2.9. Statistical Analysis
The results were stated as the mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple-comparison tests using SPSS (Version 16; SPSS, Chicago, IL, USA) was performed on the data of biochemical variables to investigate the between-group differences. The values of P < 0.05 were considered statistically significant.
3. Results
The present study describes that stevia has an influential effect on decreasing blood glucose and oxidative stress via reducing the MDA and increasing the CAT activity. The mean value of body weight, serum levels of glucose, and antioxidant status of the testis tissues in different experimental groups are shown in Table 1.
| Experimental Groups | Control (A) | Diabetic (B) | Diabetic Stevia (C) | Diabetic Metformin (D) | Healthy Stevia (E) |
|---|---|---|---|---|---|
| Body weight, gr | 284.3 ± 12.47A** | 204.7 ± 8.804B** | 261.5 ± 22.21A* | 283.5 ± 6.796A** | 269.6 ± 1.43A* |
| Serum FBS, mg/dL | 76.83 ± 3.89A** | 507.3 ± 18.91B** | 233.0 ± 61.59A** | 88.83 ± 10.04A** | 76.0 ± 5.04A** |
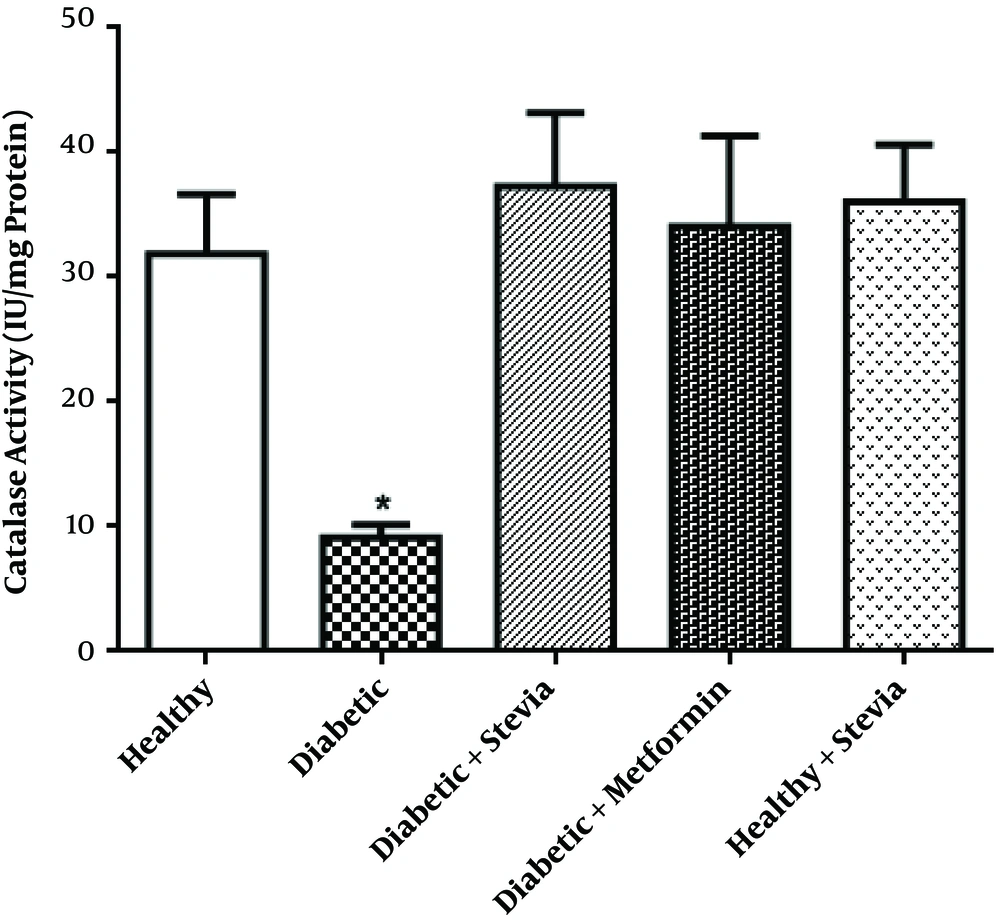
| Catalase activity, IU/mg protei | 31.89 ± 4.78A* | 9.1A1 ± 1.02B* | 37.3 ± 5.87A** | 34.08 ± 7.24A* | 36.07 ± 4.53A* |
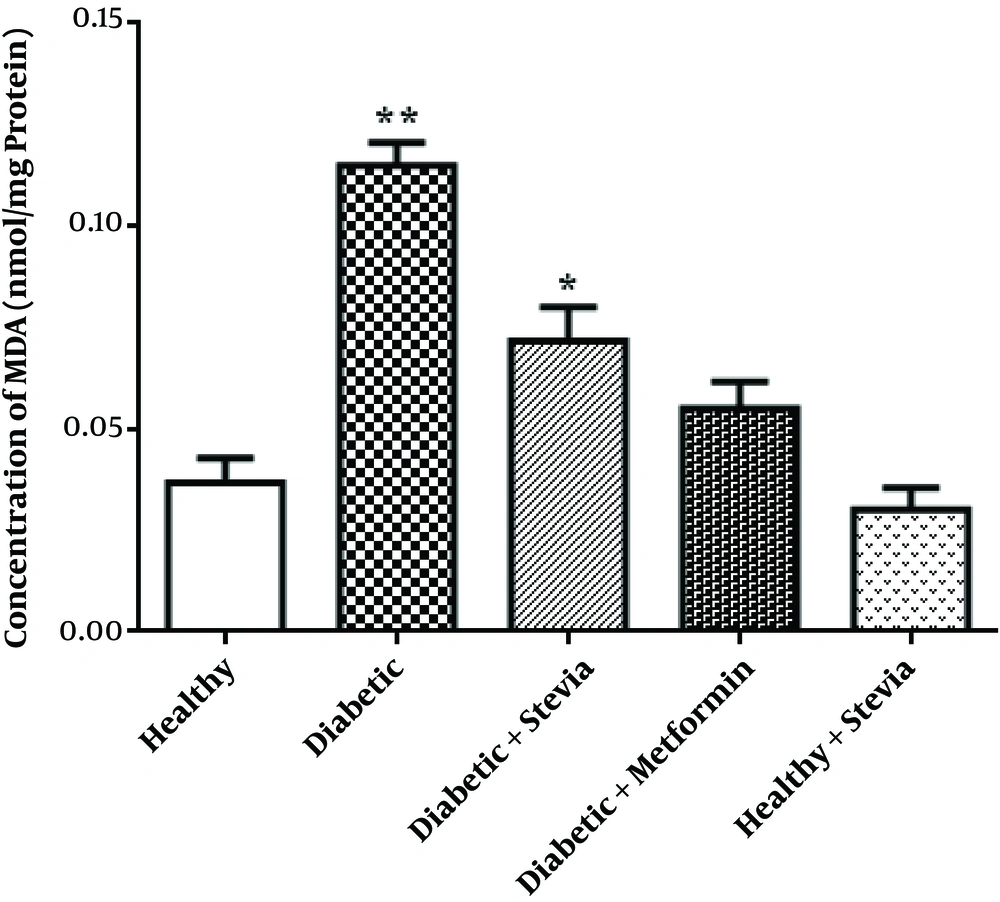
| MDA, nmol/mg protein | 0.036 ± 0.006A** | 0.115 ± 0.005B** | 0.071 ± 0.008A** | 0.055 ± 0.006A** | 0.03 ± 0.008A** |
This Table shows the increasing effect of both drugs (groups C and D) on weight compared to group B, increasing CAT activity and decreasing FBS in treatment groups, and a non-significant difference in the CAT activity and MDA level between groups A and E.
3.1. Body Weight
Body weight significantly decreased (P = 0.001) in the diabetic group (group B) in comparison with the control group (group A). In addition, in comparison with the diabetic group, body weight significantly increased in both diabetics treated with stevia (group C, P = 0.03) and diabetics treated with metformin (group D, P = 0.002) and metformin could decrease body weight more than stevia did. Between the healthy stevia (group E) and control groups, there was no significant difference (Table 1).
3.2. Serum Glucose
The level of serum FBS in the diabetic group (group B) in comparison with the control group (group A) significantly increased (P < 0.001). In both of the diabetic group treated with stevia and the metformin group (groups C and D, respectively) FBS significantly decreased (P < 0.001), but the effect of metformin in decreasing FBS was significantly more than the effect of stevia (P = 0.018). Moreover, there were non-significant differences between control and healthy stevia groups (Table 1).
3.3. Testes Catalase Activity
As shown in Table 1 the the activity of catalase in the homogenate of testes significantly decreased in diabetic rats (group B) in comparison with controls (group A) (P = 0.031). On the other hand, the activity of the enzyme increased in the groups treated with stevia or metformin (groups C and D) compared to the diabetic rats (P = 0.005, P = 0.016, respectively); the effect of stevia was better than the effect of metformin, but the differences were not significant.
3.4. Testes Lipid Peroxidation
In Table 1 and Figure 2, it has been shown that the amount of MDA significantly increased in the diabetic group compared to the control group (P < 0.001). In addition, in both groups of treated with stevia and treated with metformin, the amount of MDA significantly decreased, respectively (P = 0.001, P <0.001). Moreover, the effect of metformin was better than the effect of stevia but not significantly. Stevia had no significant adverse impact on the healthy group.
4. Discussion
The present study indicates that stevia can significantly reduce serum glucose, decline MDA level, and increase catalase activity in the testicular tissue of STZ-NA-induced diabetic rats. Indeed, these results may be due to the beneficial anti-diabetic impacts of stevia as an herbal medicine. This study showed that stevia had not any side effects; in our previous studies, this result had also been proven (19, 20).
The induction of this type of diabetes was done by the injection of NA (120 mg/kg b. wt.) 15 minutes before STZ administration (60 mg/kg b. wt.) to benefit from the effect of NA in protecting β-cells against STZ (23).
According to one of our studies conducted on STZ-NA-induced diabetic rats, after treating diabetic rats with stevia for 28 days, it was observed that stevia could decrease serum glucose but metformin was more effective than stevia in decreasing serum glucose (19). Thus, our previous outcome was in the same line and approved our present study that stevia has significant effects on decreasing serum glucose (P < 0.001). In our another study, it has been documented that stevia in STZ-induced diabetic rats after 28 days had dramatic impacts on pancreatic β-cells and raised the insulin level and diminished blood glucose by increasing PPARγ expression. PPARγ could control the level of blood glucose in diabetic rats by inducing the insulin secretion (20). Besides, in some investigations, it was revealed that stevioside not only boosted insulin secretion but also diminished gluconeogenesis in diabetic rats; therefore, serum glucose dropped (24). To sum up, stevia and its ingredients could decrease serum glucose in diabetic rats (24-27).
Hyperglycemia and insulin deficiency conditions can induce excessive reactive oxygen species (ROS) and oxidative stress in the testicular tissue (28). ROS and oxidative stress can cause functional disorders in testes and boost insulin deficiency (29, 30). Excessive free radicals or ROS lead to the depletion of the antioxidant enzymes, the drop of the testicular function, the initiation of inflammatory responses and finally, cell death (28).
In addition, by activating free radicals or ROS, lipid peroxidation happens. MDA, as a product of lipid peroxidation, increases in diabetes; it is believed that MDA amplifies the damages of oxidative stress (31). SOD preserves cells against oxidative stress by converting radicals of superoxide (O2-) into oxygen molecules (O2) and hydrogen peroxide (H2O2) (32) and CAT converts H2O2 to H2O and O2 (33). Overall, the excessive production of ROS leads to the inhibition of feedbacks and deactivation of oxidative enzymes; thus, SOD and CAT activity diminish (34).
Since stevia has emphatic effects on raising the insulin level and PPARγ expression, this plant can diminish blood glucose (20) and excess glucose will lead to oxidative stress in testicular tissue (28). It is expected that stevia can decrease excessive reactive oxygen species (ROS) in diabetic patients.
In this case, research has shown that stevia due to possessing high phenols, flavonoid, tannins enzymatic SOD, catalase, and peroxidase contents, has antioxidant properties (35, 36). In order to improve diabetic conditions and decline damages of diabetes to healthy communities, other studies demonstrate the effects of stevia and some plants on oxidative stress in diabetic patients.
In 2015, Assaei et al. reported that stevia could drop the MDA level and raise the catalase activity in the pancreas supernatant of STZ (40 mg/kg b. wt.) induced diabetic rats (20).
In 2012, Sharma et al. proved that in alloxan monohydrate-induced diabetic rats, the stevia extract had fortunate influences on decreasing lipid peroxidation and increasing CAT activity in diabetic rats’ liver within four weeks.
Besides, Singh et al. asserted that the methanolic leaf extract of stevia could improve oxidative stress in alloxan-induced diabetic mice by declining MDA during 21 days in the supernatant of diabetic rats’ liver, pancreas, and kidney.
In 2017, AbdElwahab et al. investigated the impact of the stevia leaf extract on oxidative stress in the liver tissue of alloxan monohydrate-induced diabetic rats and after six weeks, the results showed that stevia had significant effects on the MDA level in the liver homogenate.
4.1. Conclusion
Infertility in men is one of the adverse effects of diabetes. Since stevia due to its antioxidant features has a terrific effect on declining the amount of MDA and raising the CAT activity in the supernatant of diabetic rats’ testes, stevia might be effective in improving the complications of diabetes in the male reproductive system.