1. Context
The mitral valve conducts blood flow from the left atrium to the left ventricle. Mitral regurgitation occurs when the valve couldn’t be closed properly at the end of ventricular filling (1). Mitral regurgitation is the second most common valvular heart disease, preceded by aortic stenosis. The natural history of severe mitral regurgitation is a threatening problem for health without surgical intervention and may result in the worsened left ventricle, pulmonary hypertension, atrial fibrillation, and death (2).
The most common cause of mitral regurgitation is degenerative diseases, although there are a range of causes (1). The typical intervention for the mitral valve disease is surgery. It is operated under general anesthesia using the heart/lung bypass (1). This can include repairing or replacing the main valve with a prosthetic valve. There are a number of different therapeutic techniques, depending on the regurgitation mechanisms. When the valve is eligible for surgical repair, repairing the mitral valve has better outcomes than replacing it (1). About 49% of patients whose mitral valves need to be repaired or replaced are at high risk of surgical intervention; surgery is not recommended for them. These patients may relieve their symptoms through the medical management of the disease; however, this process does not modify the disease progression. Various subcutaneous techniques have been developed to treat mitral regurgitation with less-invasive approaches. Nowadays, the Mitraclip system has the most widespread clinical usage (2).
The Mitraclip system is a catheter-based device that can reduce mitral regurgitation without open surgery; this can be used for patients who are at unacceptable risk of conventional mitral valve repair or replacement (1). The entry point is generally either through the femoral vein, femoral artery, or directly through the myocardium in the apical region of the heart (3).
This study aimed to investigate safety, efficacy, and economic aspects of Mitraclip for treatment of mitral regurgitation to provide insightful evidence for health policymakers.
2. Evidence Acquisition
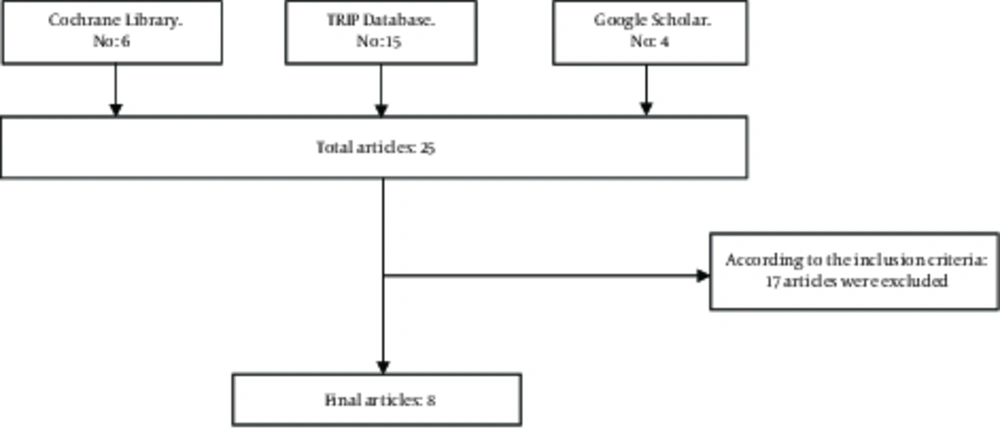
A rapid review was conducted by a systematic search of the relevant major databases. Cochrane Library, TRIP Database, and Google Scholar were searched using appropriate search strategies (Mesh terms and free texts) with no language restriction to find clinical policies, systematic reviews, economic evaluations, and health technology assessments that were up-to-date (between 2000 and January 2018) and could answer all or part of the research questions (Figure 1). The inclusion criteria were patients with mitral regurgitation that underwent Mitraclip compared with other conventional therapeutic methods including open heart surgery and exploring outcomes like 30-day mortality, recurrence (MR ≥3 +), improvement in NYHA functional class (a method of grading the extent of myocardial infarction), quality of life, one-year mortality rate, success rate, hospital length of stay, and costs. The quality of included studies and the level of evidence produced by the included studies were assessed according to the guidelines of the American College of Emergency Physicians (ACEP) (Tables 1 and 2). The exclusion criteria in this research was trial papers (RCT or CCT). All of the included papers (except of clinical policy due to lack of relevant proper checklist) were appraised in terms of quality on the base of CASP checklist. The quality of them was acceptable.
3. Results
Eight studies were included, including 3 systematic reviews, 2 economic evaluations, 2 clinical policies, and a health technology assessment (Figure 1 and Table 3).
3.1. Indications
The Mitraclip system received its pre-marketing approval from the US FDA on October 24, 2013. This approval is for the treatment of patients with mitral regurgitation and degenerative symptoms (MR ≥ 3+) (severe mitral regurgitation) who are at a high risk if undergoing surgery (3).
| No. | Study Type | Definition |
|---|---|---|
| 1 | I | A systematic review: random controlled trial (single-center and multicenter) |
| 2 | II | Clinical controlled trial (CCT), controlled studies, controlled time series studies and quasi-randomized studies |
| 3 | III | Other types of studies |
| No. | Evidence Level | Definition |
|---|---|---|
| 1 | Strong: A | • A systematic review, health technology assessment and clinical policy which were announced: there are strong documents |
| • A systematic review, health technology assessment and clinical policy which were announced: there is much evidence. | ||
| • There is at least one type I study. | ||
| • There are at least two type II studies. | ||
| 2 | Medium: B | • A systematic review, health technology assessment and clinical policy which were announced: there are middle-quality documents. |
| • A systematic review, health technology assessment and clinical policy which were announced: there is some evidence. | ||
| • There is at least one type I study. | ||
| • There are at least two type III studies with the acceptable quality. | ||
| 3 | Weak: C | • A systematic review, health technology assessment and clinical policy which were announced: there are weak, limited, very limited or unacceptable documents. |
| • There are less than two type III studies. |
| Study ID | Title | Study Type |
|---|---|---|
| 1 | NHS Commissioning Board Clinical Commissioning Policy Statement: Mitraclip (1) | Clinical policy |
| 2 | A meta-analysis of Mitraclip system versus surgery for treatment of severe mitral regurgitation (2) | Systematic review: meta-analysis |
| 3 | Surgical Management of Transcatheter Heart Valves (3) | Clinical policy |
| 4 | Percutaneous repair of mitral regurgitation with the Mitraclip (5) | Health Technology Assessment |
| 5 | A systematic review on the safety and efficacy of percutaneous edge-to-edge mitral valve repair with the Mitraclip system for high surgical risk candidates (6) | systematic review |
| 6 | A Canadian cost-effectiveness analysis of transcatheter mitral valve repair with the Mitraclip system in high surgical risk patients with significant mitral regurgitation (7) | Economic evaluation |
| 7 | EVEREST II high risk study based UK cost-effectiveness analysis of Mitraclip® in patients with severe mitral regurgitation ineligible for conventional repair/replacement surgery (8) | Economic evaluation |
| 8 | MitraClip for severe symptomatic mitral regurgitation in patients at high surgical risk (9) | Systematic review |
3.2. Complications
Potential disadvantages include heart valve surgery through catheters, a higher risk of inappropriate valve replacement, complications related to movement of catheters, and unreliable durability of the device (3) (strong evidence). There isn’t sufficient evidence regarding the safety of mitral valve repair for mitral regurgitation (1) (weak evidence). In regards to the mitral valve repair, clinical evaluations are now used to determine the long-term safety of the Mitraclip system (3) (weak evidence). Given the short-term risks (30 days), the clip has more advantages over conventional surgery; in a randomized trial, the rate of “major events of side effects” was significantly less in the clip group (15%) than in the surgical group (48%) (5) (strong evidence). During 30 days, the major side effects were observed in 3% to 38% of patients using the clip (5) (strong evidence). Patients who are at a high risk for surgical operation can receive Mitraclip for treatments with a low level of mortality and stroke risk (9) (strong evidence).
3.3. 30-Day Mortality
During 30 days, in a randomized controlled study, two patients passed away in the clip group and two patients in the surgery group.An uncontrolled randomized clinical trial reported that 1% of patients treated with the clip died within 30 days (5) (strong evidence). A 30-day mortality in patients using the clip was between 0% and 8.7% (5) (strong evidence). A 30-day mortality was not statistically significant (7.1% for the clip group versus 5.3% for the surgery group, P = 0.54) (2) (strong evidence). The mortality pooled event rates for patients who underwent of Mitraclip was 3.2% and stroke was 1.1% at 30 days (9) (strong evidence).
3.4. Recurrence Rate (MR ≥ 3 +), Improvement in NYHA Functional Class, Quality of Life and 1-Year Mortality Rate
It is possible that more patients treated with this device have experienced recurrent (MR ≥ 3 +) than patients who underwent surgery during 12 months. However, improvement in the NYHA functional class was more in the clip group. Consequences associated with better quality of life were observed in 192 patients after 1 to 12 months; generally, the one-year mortality rate varied between 10% and 24% (5) (strong evidence). There is insufficient evidence regarding the efficacy of mitral valve repair for mitral regurgitation qualitatively and quantitatively (1) (weak evidence).
Concerning mitral valve repair, clinical evaluations are used to determine the long-term effectiveness of Mitraclip system (3) (weak evidence). Nowadays, there is no good-quality or statistically significant evidence (in the UK NHS), which can prove that the Mitraclip system is beneficial in the treatment of mitral regurgitation (1) (weak evidence). Despite the higher levels of risk in Mitraclip patients compared with surgical intervention, clinical outcomes are similar, although surgery is more effective in reducing mitral regurgitation in the initial period after the intervention (2) (strong evidence). During 6 months, the mortality rate was 15% in patients treated with the clip (5) (strong evidence). Implementing Mitraclip is an option for the management of selected patients with severe mitral regurgitation who are at high risk of surgery. Current evidence suggests that Mitraclip can be used safely and effectively for these patients. Of course, for synthesizing more rigorous evidence about the efficacy/effectiveness of Mitraclip, prospective trials with medium- and long-term follow-up will be required (6) (medium evidence). There is inadequate existing evidence regarding the effectiveness and safety of Mitraclip compared to the standard treatment for patients with mitral regurgitation. Therefore, this technology is not recommended for surgical and non-surgical patients (5) (weak evidence).
3.5. Success Rate
The success rate was 72% to 100% for the patients who have been treated by the device (5) (strong evidence). Technical success for patients undergoing clip vs. surgery were 96% and 98%, respectively (P= 0.45) (9) (strong evidence).
3.6. Hospital Stay
Duration of hospital stay in patients treated with the clip was between 5 to 10 days (5) (strong evidence).
3.7. Treatment Costs
In Germany, the cost of each Mitraclip procedure is about 21,000 Euros (5).
3.8. Cost-Effectiveness
The clinical policy of UK NHS states that the present evidence is insufficient to analyze cost-effectiveness of Mitraclip (1) (weak evidence). In Canada, the ratio of incremental costs to the period of adjusted years is $23 433 in terms of quality of life. Sensitivity analysis shows that Mitraclip is 92% cost-effective compared with standard care in willingness to pay $ 50 000 per every adjusted year in terms of quality (7) (strong evidence). In Britain, Mitraclip, compared with improvement of life style in 2- and 10-year periods, has the adjusted years of 0.48 and 2.04 in terms of incremental quality. During 2 and 10 years, the ratio of incremental cost-effectiveness for Mitraclip is 52 947 and 14 800 pounds per every QALY. Mitraclip is cost-effective (8) (strong evidence).
4. Discussion
Mitraclip is among the modern medical procedures, which are still evolving even in developed countries. Generally, making use of such technologies in countries with high prevalence of cardiovascular disorders and problems (e.g. Iran) may have some advantages, which require more evidence obtained from systematic reviews alongside the trials. In a community-based perspective, what is clear is that the usefulness of this procedure can be different in populations with low and high risk of surgery. However, medical sciences tend to move towards treatments with the least (or even without) invasive procedures to decrease the side effects of surgery and health interventions; it is more important in cardiovascular diseases.
In a systematic review of 16 studies that included 2980 patients receiving Mitraclip, it was shown that if the side effect of this procedure was death, its short-term rate was low (i.e. 0.1%), while its long-term rate increased by 15.8%. It should be noted that the mortality rate as a side effect of the Mitraclip is related to the patient’s risk level; therefore, safety is significantly different in high-risk and low-risk groups (10).
It should be noted that in addition to the patient’s risks, risks of death vary according to other conditions including the presence of underlying diseases affecting the patient's condition and physiological and physical features. In another systematic review, the immediate success rate of this procedure was between 72% and 100% and the mortality rate varied between 0% and 7.8%. One-year survival rate was approximately 75% to 90%. Another positive point of Mitraclip was that the hemodynamic profile and patient’s functional status improved after it was implemented (10, 11).
The therapeutic efficacy of Mitraclip varies, depending on the progression level and extent of heart failure, patient’s condition and lifestyle, background and history of cardiovascular diseases, age, gender, and life expectancy. Therefore, clinical guidelines in developed countries, especially in Europe, carefully assess the patient's condition and his response to the therapy using clinical assessments (7).
Another problem concerning the Mitraclip procedure is economic considerations with a focus on cost-effectiveness and cost-utility in comparison with other methods, especially surgery. Few studies have been conducted in this field even in developed countries; different aspects of this issue must be analyzed with more details.
A study carried out in Canada showed that Mitraclip was 92% cost-effective in comparison with the standard of service, and the incremental cost per QALY was $23 433 in 2013. The researchers obtained the above conclusions for high-risk patients (7).
Concerning cost-effectiveness, two other studies carried out in Britain; unlike the results obtained in Canada, the results did not confirm cost-effectiveness of Mitraclip. Therefore, the National Institute for Health Care and Excellence continuously and periodically updates the evidence to understand the different aspects of this method (1, 8). The most important side effects of Mitraclip include inappropriate valve replacement, complications related to the movement of catheters, and unreliable durability of the device.
A 30-day mortality is low in patients using this device. More robust evidence are required regarding cost-effectiveness of this procedure due to the fact that the results related to cost-effectiveness are heterogeneous even in developed countries; therefore, researchers have no unique understanding of this issue. Even if there is consensus regarding the cost-effectiveness of the Mitraclip procedure, local economic assessment studies are required due to considerable differences especially in costs in Iran and other countries.
4.1. Conclusions
Owing the lack of high quality and reliable evidence about Mitraclip and its high costs, it seems that we have to wait to achieve results based on strong evidence. Nowadays, this procedure is prescribed in the research fields and, in some cases, for high-risk patients by observing strict indications, comments, and viewpoints of a team of cardiologists and after thorough and complete examinations.
4.2. Limitation of study
The authors were faced with some limitations in this research such as lack of availability of some of the included papers’ full text and inadequate number of relevant evidence.