1. Background
Management and provision of good quality drinking water contributes to reducing diseases and water borne infections in developing countries (1). Replenishable clean water sources in Nigeria are estimated at 319 billion cubic metres, with groundwater estimated at 52 billion cubic metres (2). Despite various strategies provided by the government to improve access to potable clean water in Nigeria, approximately 58% of urban and 39% of rural settings have access to clean and potable drinking water due to the rapidly growing population (3, 4). Borehole water therefore, remains an unavoidable source of potable water in Nigeria. However, contamination of ground water remains a global public health threat and related health consequences as well as chemical intoxication cannot be underestimated (5, 6). In fact, faecal contamination, domestic waste water, livestock manure, refuse dumps, and chemical pollution have been reported as sources of borehole and underground water contamination (7-9). Contaminated potable drinking water has severe health implications on humans, including diseases of the gastrointestinal tract and bacteraemia (10). Moreover, clean potable drinking water contaminated with toxic chemicals can cause acute and chronic health effects (11, 12).
2. Objectives
Potable drinking water should be free of metals/microbial contamination (13). This study therefore, aimed at assessing the overall physicochemical and bacteriological quality of potable water from selected boreholes in four crowded areas of Benin city during rainfalls. The results were compared with local and international guidelines, including National Agency for Food and Drug Administration and Control (NAFDAC), World Health Organization (WHO), and Nigeria Standard of Drinking Water Quality (NSDWQ).
3. Methods
3.1. Study Site/Sampling Design
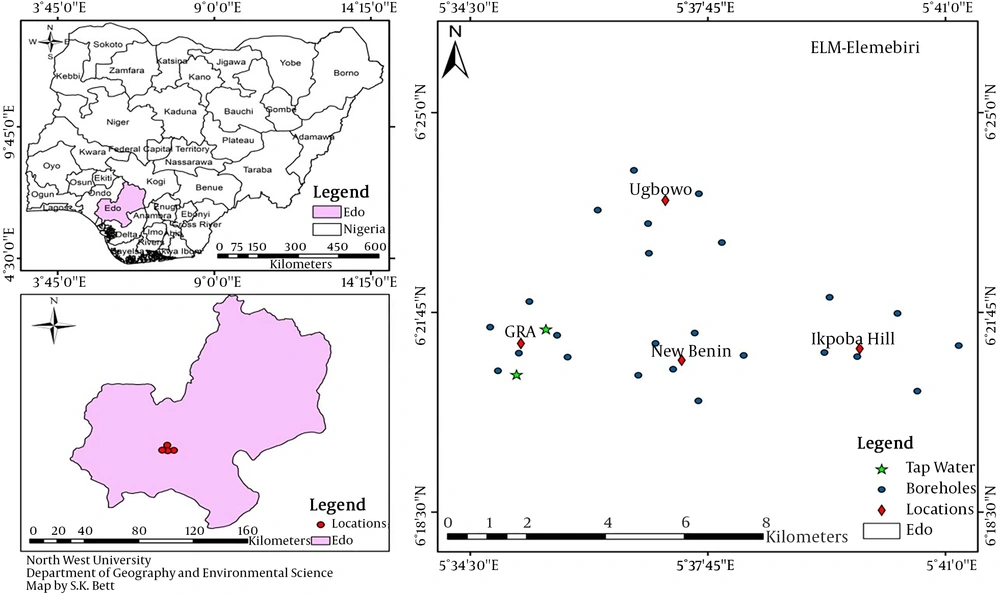
The study was conducted in Benin city, which is the capital city of Edo State, Nigeria, with an estimated population of 1495800 (4). Four major areas of Benin city namely Ugbowo, New Benin, Ikpoba hill, and GRA (Figure 1) were selected based on the availability and use of borehole water. A total of 24 borehole water samples (six samples per site) were collected from randomly selected households in four sites. In addition, two tap water samples were collected from GRA as it was the only area of Benin city with available tap water during the sampling period.
3.2. Sample Collection
Water samples were collected aseptically in 500-mL sterile Duran Schott bottles (Merck SA) and transported in ice cooler boxes to the laboratory for analysis, as described in a previous protocol (14). Samples were collected from each site, taking in consideration the water status (three treated water samples versus three untreated water samples) and the tank type (three samples from plastic storage tanks versus three samples from metallic storage tanks).
3.3. Physico-Chemical Analysis of the Water Samples
The water samples were analysed for colour, temperature, turbidity, pH, total dissolved solid (TDS), iron, chromium, nitrate, copper, and fluoride. The pH was determined using a pH meter (Model 300408-1, Denver Instruments Company, Bohemia, USA). A thermometer was used to record the temperature while the turbidity of the water was determined using a portable turbidity meter (TB200-IR-10). A UV spectrophotometer (V-1600) was used to estimate the nitrate concentration while an atomic - absorption spectrophotometer (AAS, Unicom 969) was used to determine chromium, copper, and fluoride concentrations. Iron concentration was determined using Aquachek® Iron test strips (Hach, USA).
3.4. Microbiological Analysis of the Water Samples
The Total Coliform Count (TCC) was performed using the membrane filtration technique (15). Overall, 50 mL of each water sample was filtered through 0.45-μm filter papers and the filters were placed on nutrient, mFC, and mENDO agar plates that were incubated at 37 °C for 24 to 48 hours. Blue and metallic sheen colonies from mFC and mENDO plates were purified and identified while the colonies from nutrient agar plates were purified and sub-cultured on MacConkey agar, blood agar, mannitol salt agar, cetrimide agar, and bile esculin agar plates, which were incubated aerobically at 37 °C, respectively, for 24 to 48 hours. Pure colonies were isolated and screened by gram staining and morphological/biochemical methods (16-18).
The antibiotic susceptibility test was performed for all the isolates through the disc diffusion method, as described by Jorgenson et al. (19). The antibiotic discs used included tetracycline (TET-30 μg), doxycycline (DOC-30 μg), ampicillin (AMP-10 μg), ceftriaxone (CEF-30 μg), cotrimoxazole (COT-25 μg), ciprofloxacin (CIP-30 μg), chloramphenicol (CHL-30 μg), vancomycin (VAN-30 μg), gentamicin (GEN-10 μg), and amoxicillin + clavulanic acid (AMC-30 μg) (Liofilchem, Italy).
3.5. Data Analysis
The proportion, mean and standard deviations were used to describe the contamination of the water samples. The data were compared with the WHO, NAFDAC and NSDWQ standards for drinking water. The paired t - test was used to determine if there is any significant difference at P < 0.05 between the mean TCC values of treated water and untreated water samples and between the mean TCC values of water samples from metallic tanks and that of plastic tanks. The statistical T values were calculated and compared with the critical T values. Stata 12 (StataCorp 4905 Lakeway Drive Clege Station, Texas 77845 US) was used to analyse and describe the data.
4. Results
4.1. Physico-Chemical Analysis
Table 1 shows the standard values of the physicochemical properties of clean potable water according to WHO, NAFDAC and NSDWQ as well as the means and standard deviations of the study water samples. Generally, most of the physicochemical parameters of the borehole water samples were within the recommended national and international standards, according to NAFDAC, NSDWQ, and WHO. There were traces of nitrate in the borehole water samples, justifying the nitrate values that were out of the standard limits. The mean pH value (6.84 ± 0.52) was within the recommended ranges of clean potable drinking water as shown in Table 1. The tap water sources had undetectable traces of nitrate. However, the turbidity, TDS, iron, copper, fluoride, chromium, and colour of the water samples were within acceptable limits when compared to the standards as shown in Table 1.
| Parameters | WHO | NAFDAC | NSDWQ | Sites | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Highest Desirable Level | Maximum Permissible Level | Ugbowo | New Benin | GRA | Ikpoba Hill | Tap Water (GRA) | Mean ± SD | |||
| Temperature (ºC) | - | 40 | - | ambient | 27.3 | 28.1 | 27.8 | 27.8 | 27.5 | 27.7 ± 0.3 |
| Colour (TCU) | 6 | - | 15 | 15 | - | - | - | - | - | 0 |
| Turbidity (NTU) | 5 | 25 | - | 5 | 0.80 | 0.83 | 0.79 | 0.82 | 0.76 | 0.8 ± 0.03 |
| pH | 7.0 - 8.5 | 6.5 - 9.2 | 6.5 - 8.5 | 6.5 - 8.5 | 6 | 6.7 | 7.3 | 7.2 | 7 | 6.84 ± 0 .52 |
| Total Dissolved Solid (TDS) | 500 | 1500 | 500 | 500 | 404 | 335 | 319 | 313 | 247 | 323.6 ± 56.1 |
| Nitrate (mg/L) | - | 50 | - | 0.2 | 3.6 | 4.4 | 0 | 1 | 0 | 1.8 ± 2.07 |
| Iron (mg/L) | - | - | - | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chromium (mg/L) | - | - | - | 0.06 | 0 | 0 | 0 | 0 | 0 | 0 |
| Copper (mg/L) | - | - | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fluoride (mg/L) | - | - | - | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 |
Physico-Chemical Properties of Potable Water in Benin City Compared to WHO NAFDAC and NSDWQ Standards
4.2. Microbiological Analysis
The mean TCC of all borehole water samples were above acceptable standards for drinking water. However, that of tap water samples were within acceptable limits (Table 2). The mean TCC of untreated borehole water was higher than that of treated borehole water yet there was no significant difference between the TCC value of treated water samples and that of untreated water samples as the calculated T value was smaller than the critical T value (calculated T = 0.14; critical T = 3.18). Moreover, as described in Table 2, the mean TCC of borehole water from metallic tanks was higher than that from plastic tanks yet there was no significant difference (P > 0.05) between the TCC value of metallic tank water and that of plastic tank water samples (calculated T = 0.32; critical T = 3.18).
The distribution of bacterial isolates in different water samples is described in Table 3. Out of 217 presumptive isolates, 164 bacterial isolates were identified. The most predominant isolates were Pseudomonas aeruginosa (62, 38%) followed by Escherichia coli (53, 32.3%) and Staphylococcus aureus (33, 20%), meanwhile Klebsiella pneumonia (7, 4.2%) and Enterococcus sp. (9, 5.5%) were the least common bacteria isolated. Treated water had lower microbial load and better qualities when compared to untreated borehole water. The study showed a significant difference (P < 0.05) between treated and untreated water (Table 3). Escherichia coli in drinking - water indicates faecal contamination and poor water treatment. Furthermore, E. coli, S. aureus, and P. aeruginosa were the most encountered pathogens isolated (Table 3). Samples from the tap water systems of the water board were the only samples in this study with low bacterial pathogens (Table 3).
| Parameters | TCC (CFU/100 mL) | Standards (CFU/100 mL) | Water Board Tap, (CFU/100 mL) | |||
|---|---|---|---|---|---|---|
| Water Statusa | Tank Typea | |||||
| Treated Water | Untreated Water | Plastic Tanks | Metallic Tanks | |||
| Location | 0.09 × 102 | |||||
| Ugbowo | 0.27 × 102 | 3.5 × 102 | 0.21 × 102 | 0.25 × 102 | ||
| New Benin | 0.41 × 102 | 59 × 102 | 0.3 × 102 | 4.1 × 102 | ||
| GRA | 0.36 × 102 | 5.2 × 102 | 0.16 × 102 | 0.36 × 102 | ||
| Ikpoba hill | 3.5 × 102 | 6.8 × 102 | 1.2 × 102 | 32 × 102 | ||
| Mean values | 1.13 × 102 | 18.6 × 102 | 0.47 × 102 | 9.18 × 102 | ||
| Calculated T values | 0.14 | 0.32 | ||||
| WHO | ≤ 0.1 × 102 | |||||
| NSDWQ | ≤ 0.1 × 102 | |||||
| Critical T Value | 3.18 | |||||
Total Coliform Count of the Water Samples per Location, Tank Type and Water Status
| Isolates | Location | Water Statusa | Tank Typeb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ugbowo | New Benin | Ikpoba Hill | GRA | Total Isolates | Treated Water | Untreated Water | Total Isolates | Plastic Tank | Metallic Tank | Total Isolates | ||
| Borehole | Tap | |||||||||||
| Escherichia coli | 10 | 13 | 22 | 7 | 1 | 53 (32.3%) | 23 | 30 | 53 (32.3%) | 21 | 32 | 53 (32.3%) |
| Staphylococcus aureus | 6 | 9 | 16 | 2 | 0 | 33 (20%) | 12 | 21 | 33 (20%) | 16 | 17 | 33 (20%) |
| Klebsiela pneumoniae | 7 | 0 | 0 | 0 | 0 | 7 (4.2%) | 2 | 5 | 7 (4.2%) | 3 | 4 | 7 (4.2%) |
| Pseudomonas aeruginosa | 12 | 27 | 14 | 5 | 4 | 62 (38%) | 28 | 34 | 62 (38%) | 47 | 15 | 62 (38%) |
| Enterococcus sp. | 0 | 5 | 4 | 0 | 0 | 9 (5.5%) | 3 | 6 | 9 (5.5%) | 2 | 7 | 9 (5.5%) |
| Total | 35 (21.4%) | 54 (33%) | 56 (34%) | 14 (8.6%) | 5 (3%) | 164 | 68 (41.5%) | 105 (58.5%) | 164 | 89 (54%) | 75 (46%) | 164 |
Distribution of Bacterial Isolates Based on Location, Water Status and Tank Type in Benin City
4.3. Antibiotic Susceptibility Testing
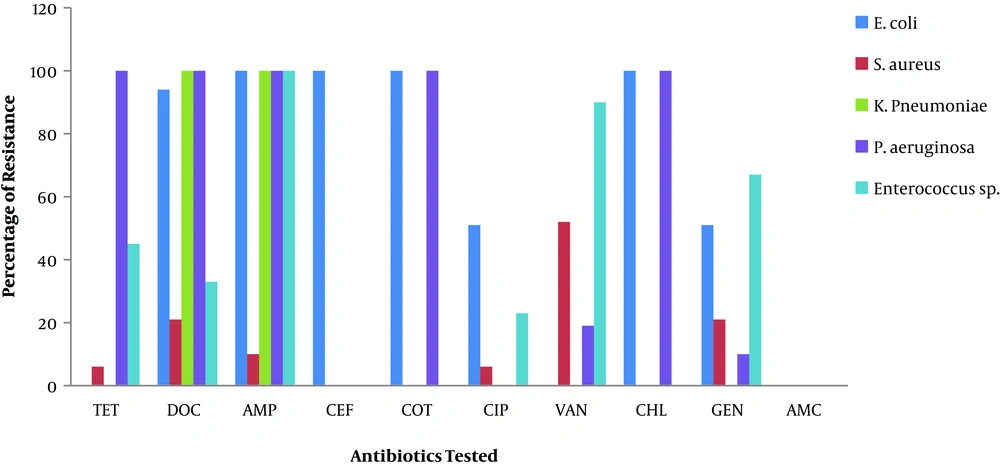
The results of the antimicrobial susceptibility test are presented in Figure 2. The majority of the bacterial isolates were resistant to doxycycline and ampicillin while no resistance was observed against amoxicillin - clavulanic acid (Figure 2). High resistance to at least five of the antibiotics was also observed among P. aeruginosa isolates while all E. coli isolates were resistant to ampicillin, ceftriaxone, cotrimoxazole, and chloramphenicol. Moreover, most S. aureus isolates showed low resistance to tetracycline and ciprofloxacin while Enterococcus sp. isolates showed the highest resistance to vancomycin.
| Isolates | Resistance Patterns | Percentage |
|---|---|---|
| E. coli | AMPRCEFRCOTRCHLR | 85 |
| S. aureus | - | - |
| K. pneumoniae | DOCRAMPR | 100 |
| P. aeruginosa | DOCRAMPR | 76 |
| Enterococcus sp. | AMPRVANRGENR | 55 |
Resistance Patterns of the Isolates
The antimicrobial resistance patterns of the isolates were determined. Two major resistance patterns were observed among the isolates and the most predominant pattern was DOCRAMPR (100%), while the least common pattern was AMPRVANRGENR (55%).
5. Discussion
5.1. Physico-Chemical Analysis
Ground water can be acidic or alkaline depending on many factors. Rainfalls are usually acidic because rain drops react with atmospheric CO2, generating acidic rainwater that percolates through organic decaying material to underground water. The pH characterization is important because biological activities can only thrive and survive within narrow pH ranges (20-23). If the soil is not rich in limestone or dolomite, the ground water will remain acidic with pH values between six and seven (23), and this might provide an explanation for the observations in this study.
Moreover, the presence of nitrates can be a source of concern because consumption of water with high nitrate concentrations can cause blood disorders (known as methemoglobinemia) as well as cancer in humans (22). These traces of nitrate could result from the close proximity of animal shelters and sewage disposal systems to boreholes, as it infiltrates in underground water after rainfalls. In fact, the oxidation of ammonia from animal and human wastes to nitrite has been proven to contaminate groundwater aquifer (24). Palamuleni and Akoth (22) also reported contamination of underground water by nitrate compounds from animal shelters in Mafikeng, South Africa.
In the present study, the physico - chemical properties of borehole water in Benin city were within acceptable limits. Similar reports were made by Mgbemena and Okwunodulu (2) in Abbia State (Nigeria), where the physico - chemical parameters of borehole water were within acceptable limits, as indicated by WHO standards.
5.2. Microbiological Analysis
Results of the present study showed that the mean TCC of all borehole water samples were above acceptable standards for drinking water (> 0.1 × 102). Proximity of some borehole water systems to waste water management systems may account for the high TCC values observed in this study. This is similar to previous findings by Palamuleni and Akoth (22), and Ugbaja and Otokunefor (7), who isolated coliforms from potable borehole water systems located near waste water sewage systems. Similar cases of microbial contamination of borehole water have been reported in Nigeria (9, 10, 13) and Cameroon (6).
Moreover, the mean TCC of untreated borehole water was higher than that of treated borehole water, although this difference was not statistically significant. It is obvious that during treatment, most microorganisms are destroyed or removed to make the water potable (25). However, the high mean TCC (1.13 × 102) for treated water observed in the present study is a serious public health concern. The presence of coliforms in treated borehole water samples may result from post-treatment contamination along the distribution line since this investigation was carried out during the rainy season. Benin city is a crowded town (population of 1495800) and is always flood - prone during rainy seasons (26) because of its poor/inefficient waste water drainage system. Moreover, New Benin and Ikpoba hill are the main commercial areas of Benin city with poor sewage/slumps and waste water disposal systems; this might affect the water quality, specifically at these two sites. For example, studies have shown that poor sewage/slumps and waste water disposal in highly dense commercial areas, especially during flooding, enhances coliforms and other bacteria to percolate and be distributed in borehole water systems (2, 26).
The presence of ferric oxide as a result of rusting can increase bacterial cultivability, especially in anaerobic conditions (27). Iron corrosion products have been reported to promote bacterial activity in water systems, thereby favouring the increase of both suspended microorganisms and biofilm - associated bacteria (27-30) as well as coliforms (31-33). This might explain the relatively higher mean TCC value (9.18 × 102 CFU/100 mL) observed in the metallic tank water samples compared with that of plastic tanks; although the state of the metallic tanks was not assessed in the present study.
The most predominant bacteria isolated from borehole water in this study were Pseudomonas aeruginosa (62, 38%) and Escherichia coli (53, 32.3%); P. aeruginosa is an important opportunistic biofilm - forming pathogen associated with contaminated domestic plumbing systems (34-36). This explains the presence of P. aeruginosa in borehole water samples. Escherichia coli is an indicator of faecal contamination; runoffs of sewage and waste water resulting from floods after heavy rainfalls contaminate underground water with faecal material (25). Although no biofilm production assay was carried out on the isolates, the protection that biofilms confer to microorganisms against disinfectants, may explain the presence of coliforms and other isolates in water samples from boreholes that had been treated (33, 36). Samples from tap water systems displayed low bacterial pathogens (Table 3). This may result from regular microbial and physico - chemical monitoring carried out by the water - board authorities.
Results of the antimicrobial susceptibility test revealed that doxycycline and ampicillin encountered the highest resistance among bacteria isolated from samples while no resistance was observed against amoxicillin - clavulanic acid. The combination of amoxicillin and clavulanic acid results in a medicine with a larger spectrum of activity, which makes it effective against bacteria that are resistant to β-lactams (37, 38). The detection of Antimicrobial Resistant (AMR) bacteria in borehole water has been reported in South Africa (8, 39), Cameroon (40), and Denmark (34). In one such study, Ateba et al. reported the presence of isolates that were resistant to chloramphenicol, vancomycin, oxytetracycline, amoxicillin, erythromycin, and sulfamethoxazole (8). The presence of AMR bacteria in borehole water has been attributed to the indiscriminate use of antibiotics, especially in animal husbandry, coupled with human practices, such as improper sanitation and discharge of human wastes in the environment (8, 41). These result in the creation of ecological niches, which serve as pools of resistance, to which bacteria can pick up antimicrobial resistance genes (41).
Meanwhile, most of the bacterial isolates in this study showed multidrug resistance to at least six antibiotics. The most predominant resistant pattern was DOCRAMPR. Multidrug resistance among members of the enterobacteriaceae family is common (42). Resistance to tetracyclines arose worldwide as a consequence of its extensive usage as a broad spectrum antibiotic and an anti-parasitic drug (43). Over time, gram positive and gram negative isolates acquired resistance attributes to tetracycline through two main mechanisms, tetracycline efflux and ribosomal protection (42).
Resistance to vancomycin was also recorded among some isolates in this survey. Vancomycin resistance in S. aureus and Enterococcus sp. is a serious public health concern especially as this drug is reserved for the treatment of severe systemic infections. Vancomycin has been used as a first choice drug for the treatment of enterococcal infections until Vancomycin Resistant Enterococci (VRE) arose. Resistance of Enterococcus was associated with the misuse of avoparcin (an analogue of vancomycin, which is used as a growth promoter) in intensive animal rearing and misuse of vancomycin in hospital settings (44, 45). Moreover, the use of glycopeptides in the management of community - acquired infections has led to the widespread of vancomycin resistant isolates in the environment (45). These studies provide an explanation of the findings.
5.1. Conclusion
Although the physico - chemical parameters of borehole water samples were within acceptable limits, the majority of the water samples were contaminated with coliforms and potential bacterial pathogens, amongst which were resistant strains. This study therefore highlights the need for continuous monitoring and quality assessment of borehole water purification processes to enhance the elimination of AMR bacteria.