1. Background
The fibrocystic breast changes are one of the most common benign lesions, which cause suffering to women. The cyclic breast pain (mastalgia) is the most common symptom of fibrocystic breast changes (1). Mastalgia causes concern and fear of breast cancer and has negative effects on quality of life (2). The concern causes excessive consumption of sedative medicines without a prescription, as well as inappropriate intervention, excessive mammograms, and undesirable, ineffective, and unnecessary self-treatment. Some evidence suggests that women with fibrocystic breast changes and women with mastalgia have a higher risk of breast cancer. In addition, fibrocystic breast changes can conceal true breast cancer (3-6). Cyclical mastalgia interferes with sexual (48%), physical (37%), social (12%), occupational and educational (8%) activities and behaviors (2, 7, 8). Joyce reports the prevalence of mastalgia in women as about 57% (9). In addition, various studies have shown the relationship between mastalgia and psychological symptoms such as depression and anxiety (2, 9).
Mastalgia medical treatments mostly include medicines with many side effects and low effectiveness, which limit the use of them (6). Today, herbal and nutritional supplements are often suggested as alternative treatments for women with moderate to severe breast pain (8). The results of a review study by Murshid indicate the effectiveness of evening primrose oil (EPO) for the treatment of cyclical mastalgia (1). EPO is rich in essential fatty acids and contains gamma-linolenic acid (GLA) as much as 7% - 14%, which is preventing the synthesis of prostaglandins that potentially cause the breast pain (10). In a study by Alvandipour et al. in Iran on periodic mastalgia, it was found that EPO is able to reduce periodic mastalgia (P < 0.001) (11). However, the study results of Salehi et al. on the effect of Vitagnus and Evening primrose on periodic mastalgia compared with vitamin E showed that EPO had fewer effects (12). In another study by Kataria et al. there was no evidence of the effect of EPO on the treatment of mastalgia (13).
Another medicine that is recommended for the treatment of mastalgia is vitamin B6. In a study by Soberiri et al. aiming to study the clinical effectiveness of vitamin B6 and vitamin E on decreasing periodic mastalgia, the trend of changes in mastalgia severity showed a significant reduction in the two groups after the intervention (P < 0.001) (6). Moreover, in a study by Soltany and Alavy Toussy it was found that vitamin B6 reduced the prevalence and the mean pain severity in periodic and non-periodic mastalgia, especially in periodic mastalgia (P < 0.01) (14). However, the results of a review study conducted by Goyal showed that vitamin B6 did not affect the treatment of mastalgia (15).
Regarding the high prevalence of mastalgia, its negative effects on the quality of life, its adverse consequences such as anxiety and depression, high medical costs due to undesirable, ineffective, and unnecessary self-treatments and also considering the contradictory and inadequate results about the effects of EPO and vitamin B6 on decreasing mastalgia, the researchers aimed to compare the effects of EPO and vitamin B6 on the severity of mastalgia associated with fibrocystic breast changes.
2. Methods
2.1. Study Design, Patients, and Setting
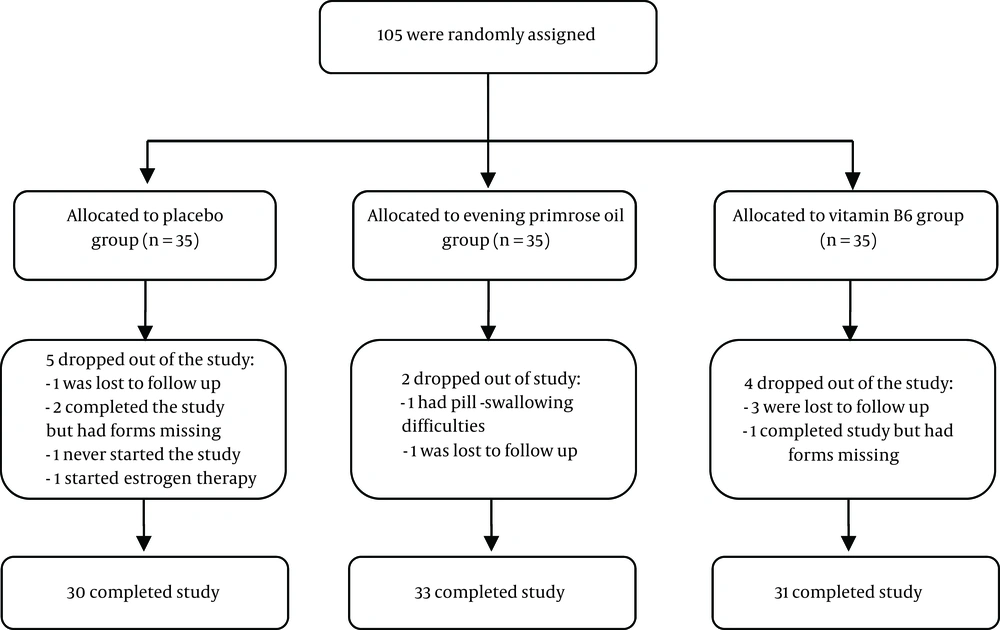
The present study is a triple blind clinical trial that aimed to compare the effects of EPO and vitamin B6 on mastalgia associated with fibrocystic breast changes. Patients referring to the breast clinic of Milad Hospital (Tehran, Iran), a referral governmental center, for treatment of mastalgia were recruited for the study from December 10, 2016, to June 10, 2017, for 6 months. The study was approved by the Ethics Committee of Iran University of Medical Sciences (reference number: IR.IUMS.REC 1395.9311373020). In addition, the study was registered at the Iranian Registry of Clinical Trials (IRCT), available at http://irct.ir/, with registration number IRCT2016062128574N1. Written informed consent was obtained from all participants. The researcher, patients, and the statistical analyzer were unaware of the allocation sequence for blinding.
The inclusion criteria included women aged 18 to 50 years with a diagnosis of fibrocystic breast changes, having moderate or severe mastalgia, no other malignant and benign breast diseases, not taking medicines for reducing pain (such as danazol, tamoxifen, bromocriptine) over the past three months, not menopause, not pregnant, and not breastfeeding. The exclusion criteria included pregnancy, physical and psychological diseases during the intervention, the consumption of any herbal or chemical sedative or hormonal medicines during the study, unwillingness to continue taking medications as prescribed, and not taking medicines for more than five days.
To determine the sample size, we used the formula of comparison of two means, following Robert et al. study (16). Using the values in this study (Mean [M1] = 17.4, standard deviation [SD1] = 4.8), taking the mean difference of at least 20%, α of 0.05, β of 0.20, power of 80%, and considering a 10% loss probability, an ideal group size of 35 was calculated for each group.
2.2. Sampling and Allocation
In this study, 94 women were selected by the simple random block allocation method based on the inclusion criteria. Allocating the participants to the experimental groups or the control group (the group taking a placebo) was done by the random allocation method using a computerized random number table and the three and six-blocking method, with an allocation ratio of 1:1:1. The allocation sequence was carried out with one individual who was not involved in the study in order to conceal the allocation. The medicines were prepared in the form of similar capsules and in cans of the same type as coded by Barij Essence Pharmaceutical Company.
Women referring to the clinic were visited by a breast disease specialist. Women eligible for the inclusion criteria would be included in the study if they were willing to participate in the study. Before the intervention, the researcher explained the research objectives to the study subjects, and if they were consent to participate in the study and signed the informed consent form, they would enter the study. At the beginning of the study, questionnaires including demographic characteristics, midwifery information, and information on breast fibrocystic changes were completed through interviews with participants. Visual analog scale (VAS) was used to evaluate the severity of mastalgia. It was a continuous scale that scored between 0 and 10; 0 indicates no pain, 1 - 3 indicates mild pain, 4 - 6 indicates moderate pain, and 7 - 10 indicates severe pain. Validity and reliability of the tool have been confirmed in previous studies (17, 18).
2.3. Intervention, Main Outcomes, and Measuring the Outcomes
The subjects were randomly assigned to the intervention and control groups: EPO (every 12 hours, one capsule of 1000 mg), vitamin B6 (every 12 hours, one capsule of 50 mg), and placebo (every 12 hours, one placebo capsule). The analysis approach of the study was per protocol (Figure 1).
Before starting the study, the researcher trained the daily recording of pain on the VAS scale to the participants. One month before the intervention and at the first, second, and third months after the intervention, mastalgia severity scores were evaluated by the VAS scale, recorded daily by the subjects. In addition, during the research, the researcher would be informed of the daily consumption of drugs and the completed questionnaires by phone calls once every week. The subjects were personally visited at the end of the first, second, and third months after the intervention to collect the filled out VAS questionnaires. At each visit, questions were also asked about the side effects and the ways of taking medicine.
2.4. Statistical Analysis
Data analysis was performed using SPSS v18 (IBM, New York, United States). The mean and standard deviation of the quantitative variables were calculated. The normality of the data was investigated using the Kolmogorov-Smirnov test. Categorical data were analyzed using the Chi-square and Fisher’s exact tests. One way analysis of variance (ANOVA) and Tukey post hoc test were used to compare between-group differences. In addition, repeated measures test and Bonferroni post hoc test were used for comparisons of the means within groups in terms of cyclical breast pain severity at different times. The main assumptions for conducting repeated measurements such as sphericity and normality were checked. Data analysis was performed at the statistical significance level of 0.05.
3. Results
The mean age in the three groups of EPO, vitamin B6, and placebo was 39.9 ± 5.85, 39.94 ± 7.40, and 40.28 ± 6.84, respectively. The mean age of menarche in subjects was 13.61 ± 1.7 and the mean body mass index (BMI) was 26.47 ± 4.63. No significant difference was observed in the demographic and fertility characteristics of the patients between the three groups (Table 1).
| Characteristics | B6 Group (n= 31) | Evening Primrose Oil Group (n = 33) | Placebo Group (n = 30) | P Value |
|---|---|---|---|---|
| Age, y | 39.94 ± 7.4 | 39.88 ± 5.8 | 40.28 ± 6.8 | 0.967b |
| Menarche age, y | 13.74 ± 1.4 | 12.53 ± 2.1 | 13.48 ± 1.6 | 0.8b |
| Education | 0.9c | |||
| Illiterate | 1 | 1 | 2 | |
| Elementary | 7 | 11 | 9 | |
| Secondary | 7 | 6 | 5 | |
| High school | 11 | 12 | 9 | |
| University | 5 | 3 | 5 | |
| Occupational status | 0.7c | |||
| Worker | 4 (13) | 6 (18.2) | 6 (20) | |
| Housewife | 27 (87) | 27 (81.8) | 24 (80) | |
| Marital status | 0.581d | |||
| Single | 3 (9.7) | 4 (12.1) | 5 (16.7) | |
| Married | 28 (90.3) | 29 (87.9) | 25 (83.3) | |
| Children number | 0.242d | |||
| ≤ 1 | 17 (54.7) | 7 ( 21.3) | 9 (30) | |
| 2 | 11 (35.3) | 16 (48.4) | 17 (56.7) | |
| 3 | 2 (6.8) | 8 (24.3) | 3 (10) | |
| > 3 | 1 (3.2) | 2 (6) | 1 (3.3) | |
| BMI | 25.98 ± 3.76 | 27.78 ± 5.19 | 25.65 ± 3.76 | 0.141b |
| Menstrual period, d | 27.03 ± 5.71 | 25.23 ± 6.42 | 27.03 ± 3.81 | 0.341b |
| Menstrual duration | 7.16 ± 1.52 | 6.40 ± 2.15 | 6.10 ± 2.05 | 0.085b |
| Regular menstrual cycle | 0.683c | |||
| Yes | 19 (61.3) | 22 (66.6) | 22 (73.3) | |
| No | 12 (38.7) | 11 (33.4) | 8 (26.7) | |
| Bleeding volume | 0.307c | |||
| Low | 0 (0.0) | 7 (21.2) | 3 (10) | |
| Moderate | 18 (58.1) | 13 (39.4) | 15 (50) | |
| High | 10 (32.2) | 9 (27.2) | 9 (30) | |
| Very high | 3 (9.7) | 4 (12.2) | 3 (10) | |
| Dysmenorrhea | 0.112d | |||
| No | 4 (12.9) | 2 (6.1) | 3 (10) | |
| Low | 3 (9.7) | 9 (27.3) | 5 (16.7) | |
| Moderate | 10 (32.2) | 10 (30.3) | 3 (10) | |
| severe | 7 (22.6) | 7 (21.2) | 13 (43.3) | |
| Very severe | 7 ( 22.6) | 5 (15.1) | 6 (20) | |
| Mother age at the first pregnancy | 22.75 ± 6.15 | 21.04 ± 4.74 | 21.81 ± 6.01 | 0.554b |
| Breastfeeding history | 0..871c | |||
| Yes | 24 (77.4) | 27 (81.8) | 26 (86.7) | |
| No | 7 (22.6) | 6 (18.2) | 4 (13.3) | |
| Fibrocystic family history | 0.307c | |||
| Yes | 11 (35.5) | 8 (24.3) | 10 (33.3) | |
| No | 20 (64. 5) | 25 (75.7) | 20 (66. 7) | |
| Tenderness | 0.443c | |||
| Yes | 28 (90.3) | 25 (75.7) | 25 (83.3) | |
| No | 3 (9.7) | 8 (24.3) | 5 (16.7) |
aValues are expressed as mean ± SD or No. (%).
bOne-way analysis of variance (ANOVA).
cChi-square Test.
dFisher’s exact test.
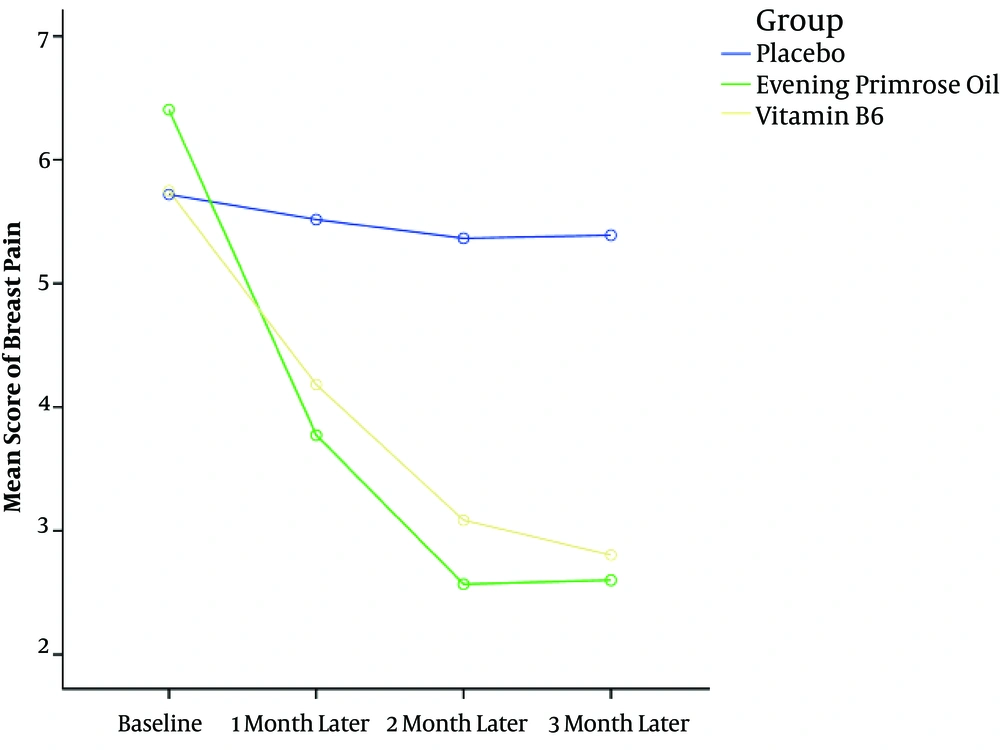
The mean breast pain severity before the intervention was 5.96 ± 2.38 and no significant difference was observed in the pain severity scores before the intervention between the three groups (P = 0.434). The mean pain score in the two groups EPO and vitamin B6 decreased significantly at the first, second, and third months after the intervention (P < 0.001) while the mean score of pain severity in the placebo group was not significantly different between before the intervention and at the first, second, and third months after the intervention (P = 0.815) (Table 2).
| Group | Baseline | The First Month After Intervention | The Second Month After Intervention | The Third Month After Intervention | within-Group Comparisons (P Value)b |
|---|---|---|---|---|---|
| B6 (n= 31) | 5.75 (2.21) | 4.18 (2.43) | 3.08 ( 2.31) | 2.81 (1.97) | < 0.001 |
| Evening primrose oil (n = 33) | 6.41 (2.36) | 3.77 (2.74) | 2.56 (2.26) | 2.61 (2.35) | < 0.001 |
| Placebo (n = 30) | 5.72 (2.58) | 5.51 (2.32) | 5.36 (2.31) | 5.38 ( 2.28) | 0.815 |
| Between-group comparisons (P Value)c | 0.434 | 0.018 | < 0.001 | < 0.001 |
aValues are expressed as mean (SD).
bRepeated measures test.
cOne-way analysis of variance (ANOVA).
Based on the repeated measures analysis, the means of breast pain severity were significantly lower in the intervention groups (EPO and vitamin B6) than in the control group (placebo) at the first, second, and third months after the intervention (P < 0.001). Moreover, the difference in the mean scores of pain severity between the two groups of EPO and vitamin B6 was not statistically significant at any intervention period (P = 0.999) (Table 3 and Figure 2).
| Comparison Between Groupsa | Baseline | The First Month After Intervention | The Second Month After Intervention | The Third Month After Intervention | ||||
|---|---|---|---|---|---|---|---|---|
| MD (95% CI) | P Value | MD (95% CI) | P Value | MD (95% CI) | P Value | MD (95% CI) | P Value | |
| B6 with Placebo | 0.3 (-1.43 - 1.49) | 0.999 | -1.33 (-2.86 - 0.192) | 0.01 | -2.27 (-3.67 - 0.88) | < 0.001 | -2.58 (-3.93 - 1.23) | < 0.001 |
| Evening primrose oil with Placebo | 0.69 (-0.77 - 2.15) | 0.76 | -1.74 (-3.27 - -0.216) | 0.02 | -2.79 (-4.19 - 1.39) | < 0.001 | -2.78 (-4.13 - -1.44) | < 0.001 |
| B6 with evening primrose oil | -0.66 (-2.11 - 0.8) | 0.826 | 0.408 (-1.11 - 1.93) | 0.999 | 0.516 (-0.882 - 1.91 | 0.999 | 0.203 (-1.145 - 1.55) | 0.999 |
Abbreviation: MD, mean difference (confidence interval).
aResults based on Bonferroni post hoc test (repeated measures).
4. Discussion
The results of the present study indicated that the mean score of mastalgia in the EPO and vitamin B6 groups significantly reduced after the intervention (P < 0.001). However, the mean score of mastalgia in the EPO group was not significantly different from that in the vitamin B6 group (P = 0.999). Salehi et al. conducted a study aiming to investigate the effect of EPO, Vitagnus, and Vitamin E on periodic mastalgia in Arak, Iran. The mean pain significantly reduced in the three groups but this reduction was more significant in the Vitagnus group (P < 0.001) (12). The results of their study are consistent with that of the present study. Nevertheless, in their study, the medicine type and the duration of the intervention (two months) were different from those of the present study. Alvandipour et al. conducted a study aiming to compare the effects of EPO and vitamin E on the treatment of periodic mastalgia in northern Iran. Mastalgia score reduced significantly in the EPO group (P < 0.001). The results of that study are consistent with that of the present study, which can be attributed to the medicine dose (two grams per day). However, in that study, the sample size was n = 25 and Evening primrose was compared with vitamin E (11). Fathizadeh et al. conducted a study to compare the effects of EPO and vitamin E on the periodic mastalgia severity. The results of that study showed EPO reduced mastalgia significantly (P < 0.05) and it was more effective and better than vitamin E (19). The result of that study is consistent with that of the present study. However, in some studies, the results are not consistent with the findings of the present study. In a study by Pruthi et al. in order to compare the effects of EPO and vitamin E on the control of cyclic mastalgia, pain severity reduction was significant between Evening primrose and placebo groups (P = 0.18) (20). The reason for this inconsistency can be due to the small sample size. In addition, the results of a clinical trial study conducted by Goyal et al. showed that EPO made no significant difference in the improvement of mastalgia compared to a placebo (21). The results of these studies are not consistent with the results of the present study (P > 0.05). Blommers et al. carried out a randomized, double-blind clinical trial study on 120 mastalgia subjects to evaluate the effect of EPO and fish oil on the severity of chronic mastalgia. In both groups, the severity of pain reduced, with a reduction of 12.3% in the EPO group and 15.5% in the fish oil group, but EPO and fish oil had statistically the same effect (22). The distinction between our study and that study is the use of a different type of medicine in comparison with EPO, which could be the cause of this inconsistency. Srivastava et al. conducted a meta-analysis of mastalgia control studies. Their results indicated that the mean score of mastalgia in the EPO group was not significantly different from that of the placebo group (3). Qureshi and Sultan conducted a study on 50 subjects in Karachi, Pakistan, aiming to compare the effects of EPO and non-steroidal anti-inflammatory ointment on the treatment of mastalgia. The result of their study showed that non-steroidal anti-inflammatory ointment was more effective than EPO in reducing mastalgia (P < 0.001). This result is not consistent with that of the present study, which may be due to the difference in the dosage of the drug used in that study and in the current study, so that the dose of the medicine was 2 g per day for all subjects in this study, while in that study, EPO was prescribed only 1 gram per day, or it could be due to the small sample size. In addition, sample selection was non-random in that study that could affect the results (23). Further studies have suggested the inability of EPO to improve mastalgia (15). Therefore, there is no strong and valid scientific evidence of the effect of EPO on the improvement of mastalgia (10, 24).
Another medicine that has been studied in the present study was vitamin B6. In a study by Soberiri et al. the severity of breast pain reduced in both groups of vitamins B6 and E at the end of the first and second months after the intervention (P < 0.001) while the results of the two medicines were statistically similar (6). Another study showed that vitamin B6 reduced the severity of periodic and non-periodic mastalgia (P < 0.01). The results of the study are consistent with the result of the present study (13). However, the results of a review study conducted by Goyal showed that vitamin B6 did not affect mastalgia (15). In addition, Smallwood J conducted a double-blind clinical trial to investigate the effect of vitamin B6 on mastalgia. The results of the study indicated that vitamin B6 was not effective in reducing mastalgia, but due to the weakness of the methodology and reporting, this study cannot be cited (15, 25). There is no credible evidence to support the use of vitamin B6 for the treatment of mastalgia (26).
The strengths of the study include a random allocation of participants to groups, allocation concealment and that, this was the first study that evaluated the effects of EPO in comparison with vitamin B6 on reducing periodic mastalgia. The potential limitation of the study was the lack of evaluation of the effects of treatment after discontinuation of the drug.
Considering the fact that no significant difference was found in the present study between EPO and vitamin B6, and given the lower price and availability of vitamin B6, it is recommended to use vitamin B6 to treat mastalgia.