1. Background
For years, it was believed that the disabled tendon tissue in adult animals is incapable of restoration; but research has proved that tendons have metabolic activity and healing ability. Orthopedic problems such as tendon laceration are quite challenging in medicine. The lower parts of the limbs are usually injured in horseracing; athletes develop tendon problems; burning accidents cause limb tendon injuries. Sometimes, the tendon gaps cannot be easily sutured; therefore, using a prosthesis or tendon graft in order to save the limb function is inevitable. Like other tissues, the tendon healing begins with an inflammatory response. The acute phase of inflammation is necessary for healing progress and fibroplasia (1).
Presence of inflammatory cells increases the fibroblastic cellularity in the onset of the healing phase. Along with the proliferation of fibroblasts, collagen production increases. Progressively, the molecular signals induce primitive collagen molecule secretion in the matrix; then, maturation adheres the collagen fibers together in the terminal phase of healing. If the healing is completely over, collagen bundles mature in the terminal phase of healing (2).
One of the important biomaterials recently used to improve healing are platelets. It is a source of growth factors in its alpha-granules. Many biomaterial products such as platelet-rich plasma (PRP) and leukocyte and platelet-rich fibrin (L-PRF) are the second-generation of platelet concentrate for different uses of soft and hard tissue injuries, burns, hard-to-heal wounds, and orthopedic conditions (2).
Platelet alpha granules contain polypeptide growth factors that stimulate the proliferation of normal connective tissue cells (3), which accelerate and initiate the inflammatory response by the host (4). They induce formation of granulation tissue by facilitating the synthesis of new extracellular matrix (ECM) and neoangiogenesis (5). Several alpha granule proteins that are released from the platelets induce direct fibroblast migration and may affect the wound healing (6).
2. Objectives
The present study aimed to compare the effects of platelet-rich plasma on tendon gap healing by using two different conduits made of subcutaneous fascia and silicone tube.
3. Methods
3.1. Surgery
This study was performed on 24 mature guinea pigs of the two sexes, weighing 750.7 ± 54.6 g. After the adaptation period which lasted one week, the pigs were prepared for an aseptic surgery. They were anesthetized with ketamine (40 mg/kg) and xylazine (5 mg/kg). An incision of three cm was created on the caudal surface of the right rear limb of each animal; the skin and connective tissue was dissected, and the deep digital flexure (DDF) tendon was isolated. A 7-mm piece of the tendon was resected to make a gap in the tendon. These resected normal tendons served as control group.
After initial preparation, they were divided into four case groups based on the surgical procedure. In the first group (G1), the subcutaneous fascia of the back of animal was dissected, separated and sutured around an IV tube to form a tube conduit. The two ends of severed tendon were sutured into the tube conduit to maintain the 7.0-mm gap between the two ends of the tendon. No PRP was injected. In the second group (G2), the tube conduit was prepared as in the first group. A 20-gauge angiocatheter was installed into the conduit, leaving its end out of the sutured skin. One milliliter of PRP was injected into the conduit and the angiocatheter was removed.
For the samples in third group (G3), pyrogen-free sterile silicone tube was used. The two ends of the severed tendon were sutured into this tube to maintain the 7.0-mm gap. No PRP was injected. In the fourth group (G4), the silicone tube was used as in group 3. A 20-gauge angiocatheter was installed into the conduit leaving its end out of the sutured skin. One milliliter of PRP was injected into the tube and the angiocatheter was removed.
The limb was protected by a temporary splint. The animals received antibiotics, anti-inflammatory and analgesic medications following the operation that continued for 3 days. The splint was removed on the tenth postoperative day. Six weeks later, the animals were euthanized by thiopental (30 mg/kg) and the tendon specimens were dissected for further study. Half of the tendon specimens were used for histopathologic study and the other half for stereological study.
3.2. Platelet-Rich Plasma Preparation
Following the anesthesia and prior to the operation, 2 mL of blood was collected from each animal in a citrated dextrose tube. The tubes were centrifuged at 1240 rpm for two minutes. The top layer which was plasma consisted of three separate layers (2:1:1 ratio from the top). The top layer was platelet-poor plasma (PPP), the middle layer was plasma average platelet (PAP) and the bottom was platelet-rich plasma (PRP). The two top layers (PPP and PAP) were removed by pipette. The third layer (PRP) was carefully separated by pipette and recentrifuged at 1240 rpm for 5 minutes. Then, the plasma layer (top layer) was discarded and the PRP layer (second layer) was collected for intra conduit injection (7, 8).
3.3. Histopathological Study
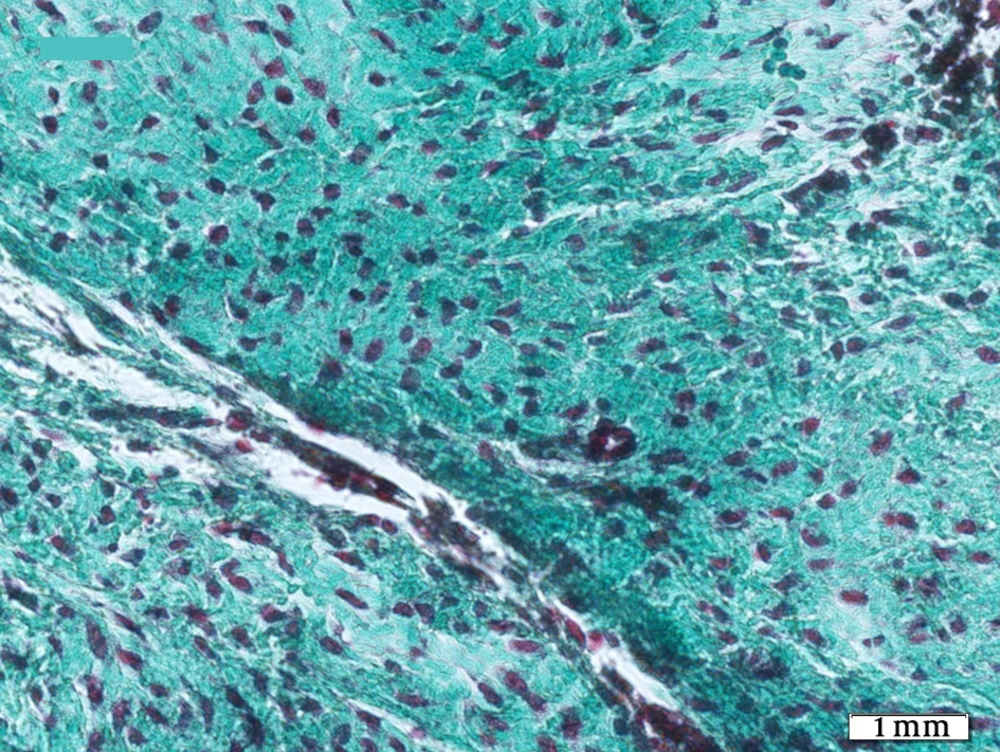
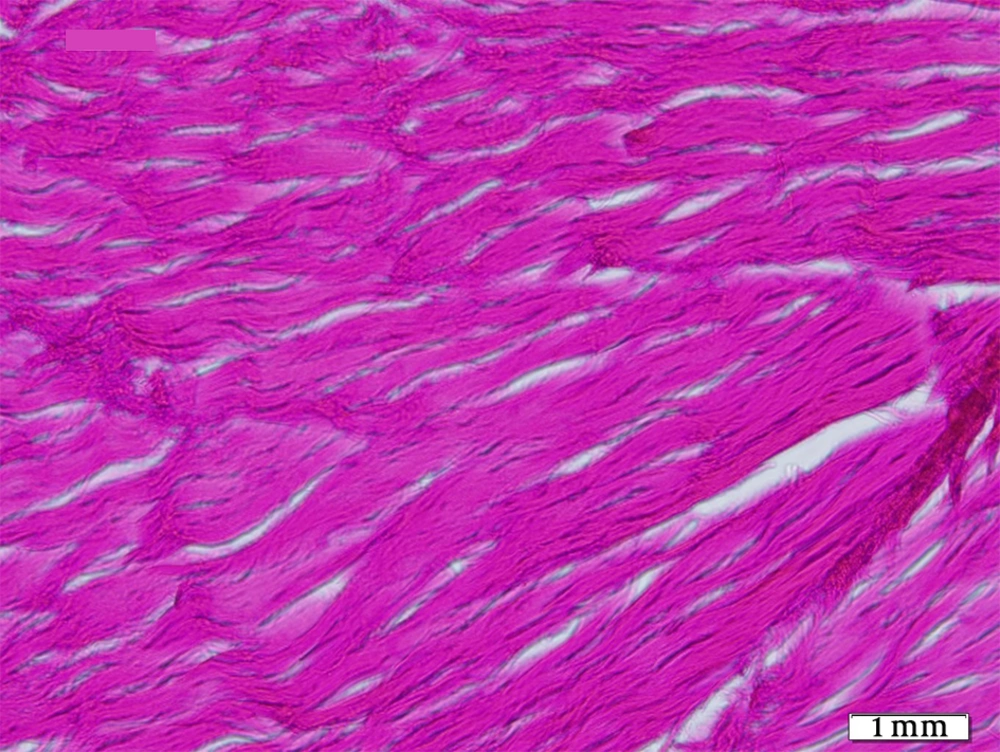
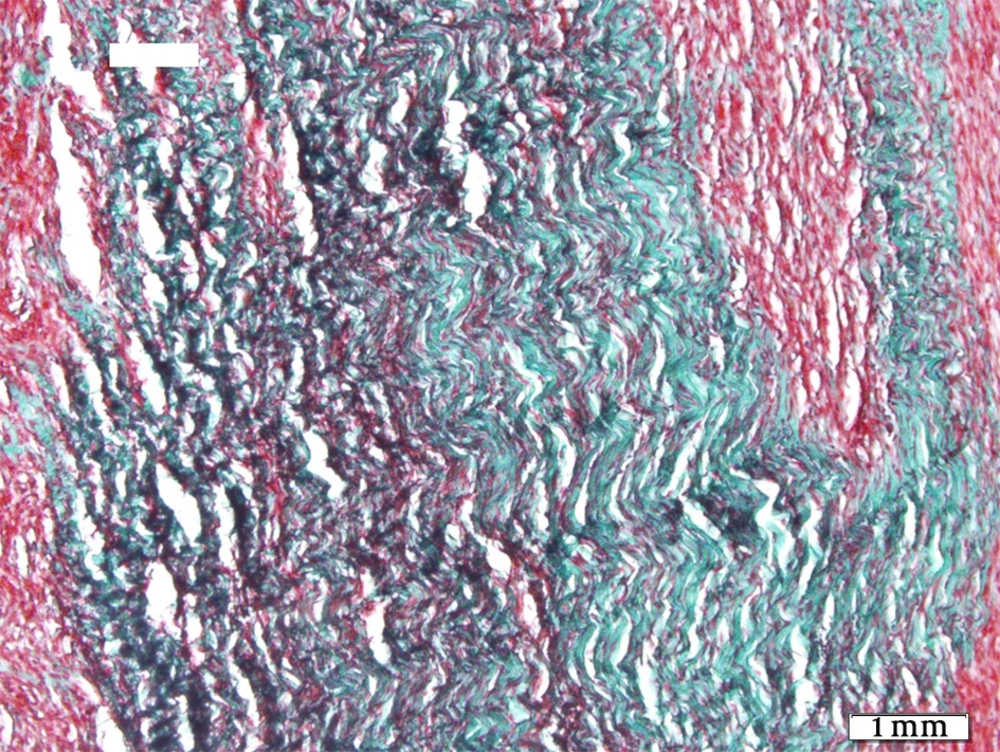
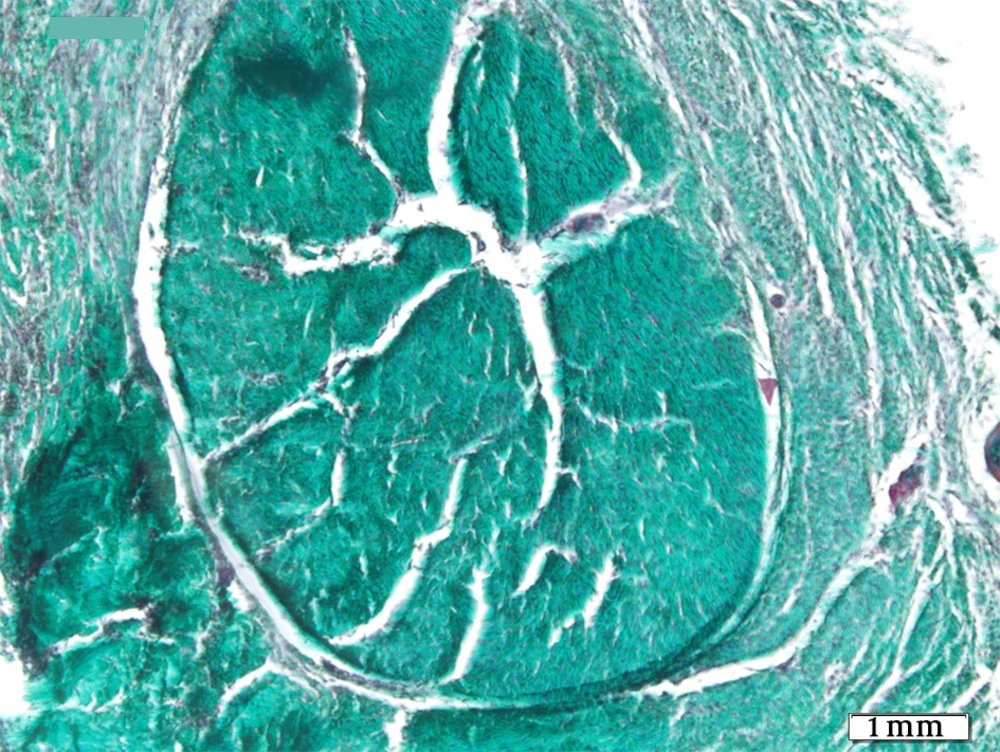
The tendons of the right rear limbs were gently removed. The samples were kept in 10% buffered formalin solution. For optical microscopy the prepared sections were divided in two parts, one part was stained by H&E and the other part was stained by Green Masson’s Trichrome staining for collagen fibers. Details such as inflammation degree, fibroblastic cellularity, collagen fiber maturation, tendon bundle formation and its diameter were surveyed in all groups and scored 1+, 2+, 3+ and - as negative.
3.4. Stereological Study
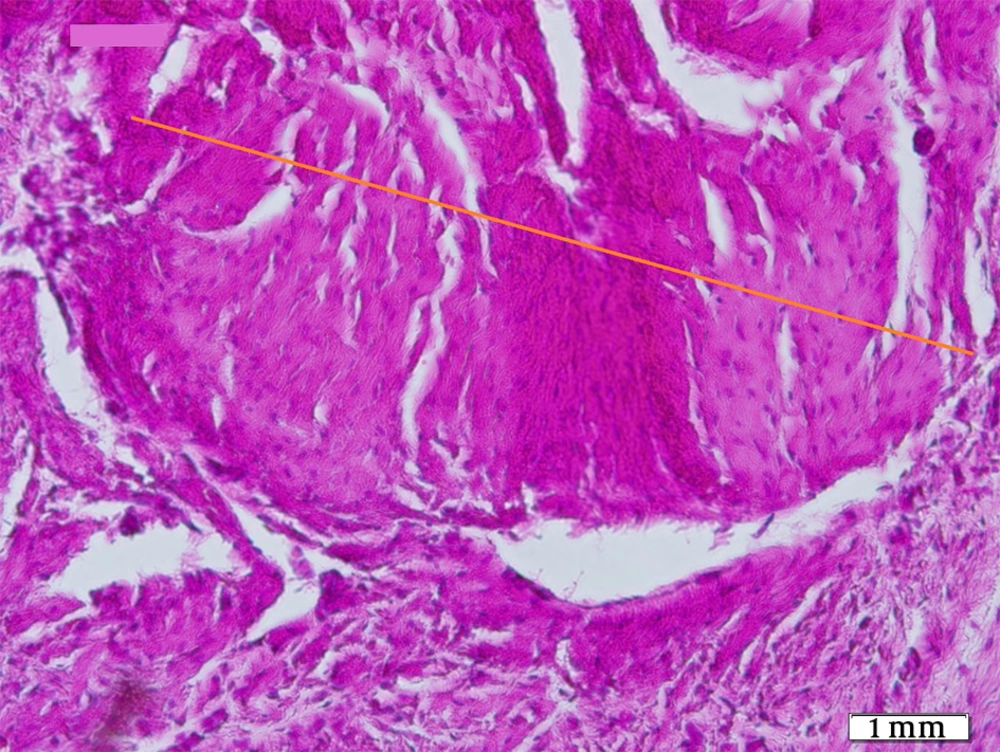
The tendons of the right rear limbs were gently removed. They were weighed by digital balance (PA224 Pioneer Lab Balance, Assembled in China). Scherle's immersion method was used to measure the primary volume (V primary) of the tendons (9-11). The orientation method was useful in order to get the isotropic random sections of the tendon (10-12). Thereafter, a random slab was punched by using a trocar in order to have an aspherical part.
Using the above-mentioned techniques provided the chance to use the upcoming formula to estimate the Degree of shrinkage (Dsh) which could possibly be caused by tissue processing and staining procedures (13, 14):
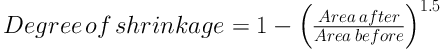
Where “area after” stood for the areas of each circular piece of the tendon before processing and staining and “area before” was the same areas after processing and staining. The tendon ultimate volume was calculated through the following formula:
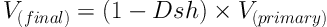
3.4.1. Estimation of the Numerical Density and Absolute Number of Fibroblast Cells
Numerical density “Nv(cells/tendon)” was evaluated by using Disector method (11). The following formula helped measuring the numerical density of the cells:
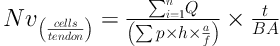
In this formula, “ΣQ” is the cell count in all the Disectors, “h” denotes the height of the Optical Disector, “a/f” is the area of the counting frame, “ΣP” is the summation of the counted frames, “BA” or block advance is the setting of the microtome to cut the paraffin block, and “t” denotes the mean thickness of the final section. The total number of the fibroblast was estimated through the following formula:
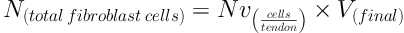
3.4.2. Measure of the Collagen Fibre Diameter
Collagen fibers diameter was measured via Olympus BX41 microscope and Olympus-DP2-PFW software.
3.5. Statistical Analysis
Kruskall-Wallis and Mann-Whitney tests were used to analyze the non-parametric data, and ANOVA and Duncan test was used for the analysis of the parametric data (SPSS software, version 16). Kolmogorov-Smirnov test was used to assess the normality assumption.
4. Results
4.1. Histopathology
Histopathological sections showed fibrotic connective tissue involving organized collagen bundles as a sign of healing in all groups (Figures 1-5). The intact tendons had no inflammation; fibroblastic cellularity was normal; collagen fiber maturation was at the maximum level, and tendon bundles were well-formed.
G1 (fascia, no PRP) showed more inflammation than G2 (fascia with PRP) and G3 (silicone, no PRP) more than G4 (silicone with PRP). G2 and G3 were the same. G1 showed the highest and G4 the lowest, significantly (P = 0.024). Fibroblastic cellularity was equal in all four case groups but statistically higher than control group (P = 0.007).
Regarding collagen fiber maturation, G2 showed more mature fibers than G1 and G4 more than G3. This maturation was highest in G4 statistically (P = 0.01) as was in control group. Tendon bundle formation was also merely equal in G1, G2 and G3 and was highest in G4 statistically (P = 0.007), but none of them reached the high level of control group.
4.2. Stereology
Tendon weight (g) was the highest in G1, decreasing by G2 and then G4 with the lowest amount in G3 and control group in fourth position. The differences were statistically significant between two later and three former groups (P < 0.05).
Regarding final tendon volume (mm3), the PRP groups with G4 on top showed more voluminosity than G1 and control group with G3 at lowest position. The difference between G4 and G3 is statistically significant (P < 0.05), while no significant difference was observed between G4 and G2.
The tendon bundle diameter (µm) was the lowest in G1, increasing by G2, then G3 with G4 at maximum level in case groups; But none reached the highest amount in control group. The difference between G1 and control group was statistically significant (P < 0.05). There were no significant difference between G1 and G2, G3 and G4, and G2 and G4.
Fibroblast cell count also showed high amounts in PRP groups with G2 at top, followed by G1, then G3 as the lowest count between case groups. The control group was the minimum of all. The latter two groups were significantly different in comparison with top three ones (P < 0.05).
5. Discussion
Tendons are composed of a hypo-cellular and hypo-vascular fibrous collagen tissue with a slow healing rate (15). There are several surgical procedures and techniques for dealing with tendon injuries. Like other body tissues, tendon healing begins with inflammatory reactions. Acute phase inflammation is essential for the restoration and fibroplasia; since the severe inflammatory reaction accelerates the healing of wounds (4); But it seems a booster is needed to improve the healing, hence the study compared the effects of platelet-rich plasma on tendon gap healing by using two different conduits made of subcutaneous fascia and silicone tube.
Histopathological data showed fibrotic connective tissue and bundle formation as a sign of healing in all groups go along intact tendons (control group) with no inflammation, normal fibroblastic cellularity, mature collagen fibers and well-formed tendon bundles. All case groups had inflammation. G1 showed the highest and G4 the lowest. PRP groups were less inflamed than non-PRP equivalents. PRP and other platelet derivatives are proven to have microbiocidal properties, this may help in not calling inflammatory cells (16, 17). Inflammation is likely to degrade the intact collagen and destroy the viable cells, consequently exacerbating the functional deficit and prolonging the recovery period. On the flipside, the inflammatory responses include a series of cellular and sub-cellular events, which contribute to the release of growth factors, and help the healing process (15). This justifies the presence of inflammation in all groups and that PRP may decrease the destructive aspect of inflammation (7).
The study showed that fibroblastic cellularity was equal in all four case groups but statistically higher than control group. This means the high proliferative activity is needed in repair processes for fundamental cells. Tendon repair is a complex and highly regulated process, whose initiation, maintenance and cessation depend on a large number and variety of molecules. Growth factors are one of the most important molecular families involved in the healing process (18). Platelets are produced in the bone marrow and have growth factors, which are the main factor in healing and tissue repair. The growth factors are stored in alpha granules, and when released, they induce cellular growth, proliferation, and differentiation in different cells. Thereby, this event increases fibroblast proliferation, collagen synthesis, intracellular matrix formation, vascularity, thickness, and tissue healing (19).
Regarding collagen fiber maturation, G2 showed more mature fibers than G1 and G4 more than G3. This maturation was highest in G4 statistically as was in control group. The results confirmed better maturation in PRP groups and that silicone is a better conduit as a surgical technique. Formation of the granulation tissue would be easier through the chemotaxis of monocytes, neutrophils, fibroblasts and myofibroblasts, as well as the synthesis of new extracellular matrix and neoangiogenesis that follows the platelet-induced hemostasis and release of the growth factors such as transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor (PDGF) (19).
The main role of growth factors is to increase the cell survival and proliferation, which are important in rebuilding and repairing the tissue (20). Growth factors stimulate cell migration, differentiation, and contractility, and additionally improve the production of specific proteins such as collagen in fibroblasts. Tendon healing is mostly done by TGF-β, PDGF, insulin-like growth factor (IGF-1), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF). TGF-β affects the regulation of cellular migration, proliferation, and fibronectin binding interactions (18).
Tendon bundle formation was also merely equal in G1, G2 and G3 and was highest in G4 statistically, but none of them reached the high level of control group. The mechanism may be due to PDGF, which participates in tissue remodeling and is produced upon the tendon damage and triggers the production of other growth factors such as IGF-I, all leading to tissue maturation (21) by affecting fibroblast secretion of collagen type III first, and later collagen type I when they go to the wound bed. Therefore, collagen production are spatially controlled to form bundles (19), so the results show the same mechanism in all groups and suggest silicone approach.
Fibroblast cell count showed high amounts in PRP groups with G2 at top, followed by G1, then G3 as the lowest count between case groups. The control group was the minimum of all. Silicone tube conduit had the least fibroblast cell count among the four case groups. However, this factor increased in the combination of both silicone tube conduit and PRP, indicating the effective role of a growth factor pool in repair processes as confirmed by review of literature (1, 5, 22).
Tendon weight was highest in G1 and PRP groups were significantly heavier than G3 and control group. The tendon weight follows the number of cells and the tissue water content. Therefore, the greater the total cell count and tissue water content is, the more the tendon weight would be (15). These stereological findings confirm the histopathological observations. Regarding the other stereological parameters examined in this study, PRP injections as well as silicone tube affected the tendon bundle diameter and final tendon volume. The tendon bundle diameter was the lowest in G1, increasing by G2, then G3 with G4 at maximum level in case groups; while none reached the highest amount in control group, indicating that the healing process was not yet completed in the 4 groups after 6 weeks and required longer follow ups. Also, tendon volume was high in PRP groups with G4 at the top showing more voluminosity than G1 and control group with G3 at lowest position. The silicone conduit was more effective than the fascia, it may be that silicones also not allow microbiological growth (23).
All the histopathological and stereological data gathered in the present study showed that PRP injection significantly improved the tendon gap healing. Certainly, the synergistic effect of both PRP and silicone conduit was much more considerable. Other studies recommend using PRP or other platelet derivatives as a regenerative medicine strategy for the treatment of injured tissues (20, 24), so does this study.
5.1. Conclusions
The present study found that PRP improved healing of tendon injury and silicone conduit served as good surgical technique with synergistic effect alongside PRP.