1. Background
Carpal tunnel syndrome (CTS) is an entrapment neuropathy accounting for 90% of peripheral neuropathies with higher prevalence in women (1-4). Damage to the median nerve in the carpal tunnel due to increased pressure leads to CTS (5).
Various treatment approaches are available for CTS, including surgical and non-surgical interventions (6, 7). Local corticosteroid injection is widely used to reduce the severity of the symptoms and improve the functional status of affected hands (4, 8-10). This procedure is often performed blindly using anatomical landmarks, resulting in complications such as damage to the median nerve and surrounding vessels and tendons. Moreover, the injection site of the steroid may not be exactly in the carpal tunnel, leading to complications such as fat tissue atrophy and skin color changes. Although these complications are very rare, it is important to inject accurately into the carpal tunnel (11, 12).
Ultrasound (US) can be used to guide a successful injection within the carpal tunnel, reduce patient discomfort, and decrease median nerve injury (13). However, the US-guided injection has additional costs for the patient and is not available in all clinical settings (14).
Ustun et al. compared US-guided versus blind corticosteroid injection for the treatment of CTS. They found that the improvement in the symptom severity scores (SSS) at week 12 was significantly higher and the average time to improvement was shorter in the US-guided group than in the Landmark (LM)-guided group. However, there were no significant differences between the two groups in other parameters (15). In another study by Eslamian et al., no statistically significant difference was observed between US-guided and LM-guided CTS injection (16). There are also other studies comparing US-guided and LM-guided corticosteroid injection for the treatment of CTS, with conflicting results (17).
2. Objectives
Because one of the limitations of the previous studies is the limited sample size, the aim of the current study was to evaluate the degree of symptom improvement, safety, and the change of electrophysiological findings in US-guided versus LM-guided local steroid injection with relatively large sample size.
3. Methods
3.1. Study Design
The present study was a randomized clinical trial comparing US-guided and LM-guided injection of Methylprednisolone 40 mg into the carpal tunnel of patients with moderate and severe idiopathic CTS.
Written informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of Isfahan University of Medical Sciences, approval code 395625, and conducted in compliance with the Helsinki Declaration. The trial was registered at the Iranian Clinical Trial Registry with identification number “IRCT2017010831833N1”.
3.2. Study Population
Patients suffering numbness, paresthesia, or pain in the median nerve territory referred to the Electrophysiology Clinic of Physical Medicine and Rehabilitation Department of Isfahan University of Medical Sciences to undergo an electrophysiological study. The sample size was determined statistically using the results of previous similar studies (15) with a 95% confidence interval and a power of 80% (18).
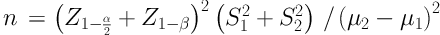

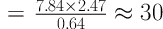
α = 0.05
1 - β = 0.8
S1 = 1.05
S2 = 1.17
µ2 - µ1 = 5.89 - 5.09 = 0.8
Therefore, 60 patients with moderate or moderate-to-severe CTS were recruited and randomly assigned to two parallel groups, LM-guided and US-guided injection (30 patients in each group). The group assignment was conducted using simple random allocation by RANDLIST 1.2 software.
Moderate CTS was defined as abnormal latency of the median sensory nerve and prolongation of median distal motor latency. Moderate-to-severe CTS was defined as prolonged median motor and sensory distal latencies, with either low-amplitude SNAP or mixed nerve action potential (NAP), or low-amplitude CMAP without any fibrillations, reduced recruitment, or motor unit potential changes in needle EMG (19).
The inclusion criteria were: (A) subjects with CTS symptoms, demonstrating positive Tinel’s sign, Phalen, and compression tests, (B) having moderate-to-severe CTS according to the electrodiagnostic criteria, (C) surgery refusal, (D) age of older than 18 years, and (E) agreement with corticosteroid injection.
The exclusion criteria included pregnancy, secondary CTS due to metabolic disorders such as thyroid disease, diabetes mellitus, rheumatologic disorders, chronic kidney disease, and wrist fractures, a history of corticosteroid injection, and conditions mimicking CTS, such as cervical radiculopathy, brachial plexopathy, polyneuropathy, and thoracic outlet syndrome, a previous wrist surgery, physical or medical therapy in the previous month, thenar muscle atrophy, and patient’s refusal to complete the follow-ups.
3.3. Injection Techniques
In the anatomic LM-guided group, the injection was done using the ulnar side approach from medial to Palmaris longus tendon. The subjects were placed in a comfortable supine position while the forearm was supinated and the wrist was in a slight dorsiflexion position. After skin preparation and antisepsis, a 26-gauge needle was inserted at an angle of 30 degrees and to the depth of 5/8 inches (the length of the needle) at the proximal to the distal wrist crease just medial to the palmaris longus tendon.
The in-plane ulnar approach was used for the US-guided injection technique for CTS treatment as precisely described by Smith et al. (20). The intervention was performed using a commercially available Sonographic scanner (Sonosite SII, Fujifilm Sonosite, Inc. USA), 6.0 to 13-MHz linear transducer.
In both groups, methylprednisolone acetate 40 mg (Depo-Medrol, injectable suspension, USP) was used without local anesthetics. Standard wrist splint with 0 - 5 degrees of hand extension at night, gabapentin 300 mg daily, and vitamin B1 300 mg daily were administered for both groups during the study.
All the electrodiagnostic studies were done by the same investigator. A Medelec Synergy (Viasys, Ireland, 2008) electromyography was used for electrodiagnostic studies.
3.4. Outcome Measurement
The outcomes were evaluated using clinical and electrophysiological parameters measured at baseline, four, and 12 months after the injection. Electrophysiological parameters included distal motor latency (DML) (21), compound muscle action potential (CMAP) amplitude (mV, recorded in the abductor pollicis brevis (APB) muscle), sensory nerve action potential (SNAP) amplitude (μV), and SNCV (m/s) recordings from digit III, and sensory latency. The Boston carpal tunnel symptom questionnaire and function assessment scale (BCTQ) were used for clinical assessment. The outcome measurements were done by a resident of physical medicine and rehabilitation who was blind to groups.
3.5. Statistical Analysis
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) 16.0 software for Windows (SPSS Inc., Chicago, USA). The differences in clinical and electrophysiological findings between baseline and post-treatment stages in each group were evaluated by the Friedman test (all pairwise comparisons made by Wilcoxon signed-rank test). The normality of variables was assessed by the Kolmogorov-Smirnov test. The differences in clinical and electrophysiological findings between the two groups were analyzed by t-test if variables were normal and by nonparametric Man-Whitney U test if variables were not normal. Moreover, ANCOVA (Analysis of covariance) and non-parametric ANCOVA (Quade’s rank) adjusted for baseline as a covariate were applied when the two groups were statistically different at baseline.
4. Results
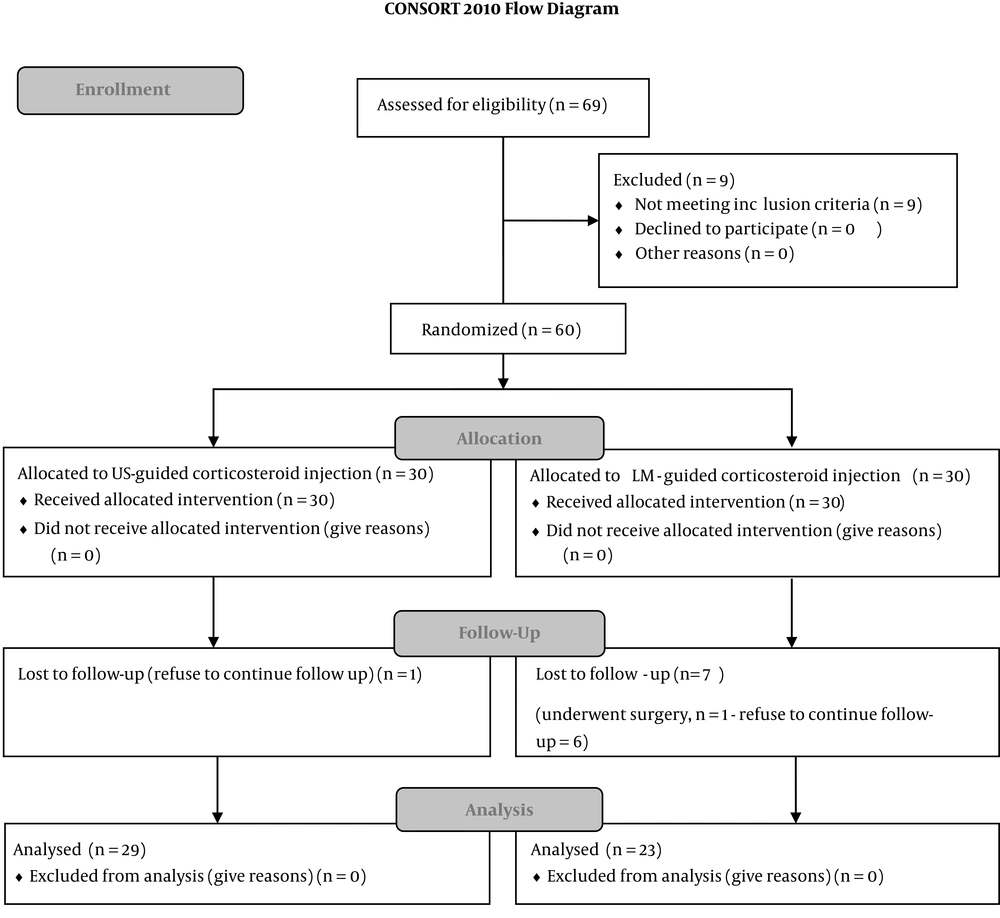
The study was conducted on 60 subjects (46 women, 14 men) with moderate or moderate-to-severe CTS who were randomly divided into two groups. During the study, one patient in the LM-guided group underwent surgery; one patient in the US-guided group and six patients in the LM-guided group did not complete the follow-ups; therefore, the eight patients were excluded from the study (Figure 1).
Of 52 patients remaining (29 in the US-guided and 23 in the LM-guided groups), 12 were men (23.1%) and 40 were women (76.9%), with the age range of 30 to 65 years. There were no differences between the two groups in age, gender, CTS grade, SSS, SNAP amplitude, and CMAP amplitude at baseline (P value > 0.05). However, other parameters were different at baseline (P value < 0.05) and special tests were used for their analysis. The baseline comparison of the two groups is shown in Table 1.
| Characteristics | US-Guided (N = 29) | LM-Guided (N = 23) | P Value |
|---|---|---|---|
| Age | 48.14 ± 9.41 | 47.61 ± 8.30 | 0.833 |
| Gender | 0.553 | ||
| Male | 7 (24.1) | 5 (21.7) | |
| Female | 22 (75.9) | 18 (78.3) | |
| CTS grade | 0.213 | ||
| Moderate | 23 (79.3) | 21 (91.3) | |
| Severe | 6 (20.7) | 2 (8.7) | |
| SSS | 2.99 ± 0.94 | 2.73 ± 0.85 | 0.315 |
| FSS | 2.68 ± 0.81 | 2.04 ± 0.92 | 0.011 |
| SNAP latency | 4.89 ± 0.77 | 4.49 ± 0.43 | 0.029 |
| SNAP amplitude | 13.90 ± 7.96 | 17.57 ± 8.44 | 0.115 |
| SNAP NCV | 26.14 ± 7.23 | 30.68 ± 5.29 | 0.015 |
| CMAP latency | 5.83 ± 1.35 | 5.78 ± 1.06 | 0.005 |
| CMAP amplitude | 2.21 ± 0.41 | 2.08 ± 0.28 | 0.897 |
aValues are expressed as No. (%) or mean ± SD.
In this study, the differences in the SNAP and CMAP amplitudes were statistically significant between the US-guided and LM-guided groups at four and 12 weeks after treatment. Although the improvement in the CMAP amplitude at week 12 was more in the US-guided group than in the LM-guided group (P value = 0.002), the SNAP amplitude and SNAP NCV were more significantly improved in the LM-guided group. There were no significant differences between the US-guided and LM-guided groups in the SSS, functional status scale (FSS), and CMAP latency at the 4th and 12th weeks of treatment (P value > 0.05) (Table 2).
| Scores/Group | Week 4 | Week 12 | ||
|---|---|---|---|---|
| Mean ± SD | P Value | Mean ± SD | P Value | |
| SSS | 0.274a | 0.985a | ||
| US | 1.50 ± 0.43 | 1.47 ± 0.62 | ||
| LM | 1.44 ± 0.50 | 1.47 ± 0.50 | ||
| FSS | 0.389b | 0.585b | ||
| US | 1.38 ± 0.47 | 1.39 ± 0.60 | ||
| LM | 1.17 ± 0.27 | 1.37 ± 0.53 | ||
| SNAP latency | 0.034c | 0.363c | ||
| US | 4.47 ± 0.55 | 4.36 ± 0.55 | ||
| LM | 4.04 ± 0.40 | 4.05 ± 0.48 | ||
| SNAP amplitude | 0.008d | 0.016d | ||
| US | 17.81 ± 7.88 | 17.56 ± 7.25 | ||
| LM | 24.23 ± 8.88 | 23.28 ± 9.39 | ||
| SNAP NCV | < 0.001c | 0.010c | ||
| US | 30.30 ± 5.14 | 30.86 ± 5.14 | ||
| LM | 37.91 ± 6.47 | 36.79 ± 6.08 | ||
| CMAP latency | 0.873b | 0.739b | ||
| US | 4.77 ± 0.64 | 4.64 ± 0.50 | ||
| LM | 4.36 ± 0.28 | 4.33 ± 0.44 | ||
| CMAP amplitude | 0.039a | 0.002a | ||
| US | 6.98 ± 1.36 | 7.01 ± 1.20 | ||
| LM | 6.37 ± 1.66 | 6.21 ± 1.78 | ||
aMann-Whitney test.
bNon-parametric ANCOVA test adjusted for baseline (Quade’s rank test).
cANCOVA test adjusted for baseline.
dIndependent t-test.
Changes in both clinical and electrophysiological findings were also examined. The SSS, FSS, SNAP latency, and CMAP latency in both groups and the CMAP amplitude in the US-guided group significantly reduced at the 4th and 12th weeks compared to baseline (P value < 0.001) (Table 3). While there was an increase in the CMAP amplitude in the LM-guided group at the 4th and 12th weeks, these changes were not significant using post hoc analysis (P value = 0.074 and 0.159, respectively; Wilcoxon signed-rank tests). Moreover, no significant changes were observed in any of the clinical and electrophysiological findings between the 4th and 12th weeks of the study (P value > 0.05).
| Scores/Group | Baseline vs. Week 4 | Baseline vs. Week 12 | Week 4 vs. Week 12 | |||
|---|---|---|---|---|---|---|
| Mean Difference | P Value | Mean Difference | P Value | Mean Difference | P Value | |
| SSS | ||||||
| US | -1.49 | < 0.001 | -1.52 | < 0.001 | -0.02 | 0.077 |
| LM | -1.29 | < 0.001 | -1.28 | < 0.001 | 0.03 | 0.823 |
| FSS | ||||||
| US | -1.30 | < 0.001 | -1.28 | < 0.001 | 0.01 | 0.511 |
| LM | -0.87 | 0.015 | -0.67 | 0.001 | 0.20 | 0.417 |
| SNAP latency | ||||||
| US | -0.43 | 0.001 | -0.53 | < 0.001 | -0.10 | 0.974 |
| LM | -0.45 | < 0.001 | -0.44 | < 0.001 | 0.01 | 0.712 |
| SNAP amplitude | ||||||
| US | 3.91 | < 0.001 | 3.65 | < 0.001 | -0.25 | 0.646 |
| LM | 6.66 | < 0.001 | 5.71 | 0.030 | -0.96 | 0.421 |
| SNAP NCV | ||||||
| US | 4.16 | < 0.001 | 4.72 | < 0.001 | 0.56 | 0.948 |
| LM | 7.23 | < 0.001 | 6.11 | < 0.001 | -1.12 | 0.606 |
| CMAP latency | ||||||
| US | -0.49 | < 0.001 | -0.63 | < 0.001 | -0.13 | 0.793 |
| LM | -0.33 | < 0.001 | -0.37 | < 0.001 | -0.03 | 0.883 |
| CMAP amplitude | ||||||
| US | 1.15 | < 0.001 | 1.18 | < 0.001 | 0.03 | 0.997 |
| LM | 0.59 | 0.074 | 0.43 | 0.159 | -0.16 | 0.322 |
The LM-guided group showed a regress in all variables except for the CMAP latency at week 12 compared to week 4; however, these improvements persisted at week 12 post-treatment in the US-guided group (P > 0.05), except for the FSS and SNAP amplitude.
There were no complications associated with the injection of corticosteroid in the carpal tunnel in both groups.
5. Discussion
Although diagnostic ultrasound and sonographic guidance are increasingly being integrated into musculoskeletal clinics and are used for carpal tunnel injection, there are some controversies between the results of recently published studies (15-17).
The primary aim of this study was to compare the efficacy and safety of US-guided versus LM-guided corticosteroid injection in CTS patients.
Corticosteroid injection is known as a safe, effective treatment for the temporary relief of symptoms associated with CTS; it can also cause a significant improvement in electrophysiological parameters (6, 8). In agreement with previous studies, the results of the current study showed both US-guided and LM-guided method of corticosteroid injection led to significant improvements in clinical and electrophysiological findings at the 4th and 12th weeks after treatment compared to the baseline. In the US-guided group, all parameters continued to improve to the 12th week, whereas in the LM-guided group, some parameters showed a regression at the 12th week.
McNally et al. reviewed the basic procedure for US-guided injection into the carpal tunnel. Grassi et al. described a classic US-guided approach for injection into the carpal tunnel and reported that improvements increased over a period of weeks. Smith et al. reviewed various US-guided injection approaches into the carpal tunnel and described a new transverse imaging method using an in-plane ulnar approach, which was also utilized in the current study (20, 22).
The first randomized controlled trial was done by Ustun et al. in which, faster and better improvement of symptom relief was observed in the US-guided group than in the blind group. They did not compare groups in terms of electrophysiological findings (15). In this study, the SSS was not significantly different in either the 4th or 12th week.
Lee et al. observed a significant improvement in DML in the US-guided in-plane ulnar group after 4 and 12 weeks, but no significant improvement was seen in DML in the US-guided out-plane ulnar and the blind group. The median nerve CMAP amplitude and sensory latency significantly improved in the US-guided in-plane ulnar and out-plane ulnar groups but not in the blind injection group. The median nerve SNAP amplitude, SSS, and FSS increased significantly in all the three groups. They did not report between-group comparisons (23). An improvement was observed not only in SSS and FSS but also in electrophysiological findings in both groups, which contradicts the Lee et al. study.
In a recent study conducted by Eslamian et al., a significant difference was not observed between the US-guided and LM-guided injections for CTS treatment, which is similar to the current study findings (16). No major adverse events were noted in the present study, in line with previous studies (15, 23).
The sample size was larger in the current study than in all previous studies. However, longer follow-ups and larger sample sizes are needed for better evaluation of long-term effects of the US-guided injection.
5.1. Conclusions
The present study primarily addressed the effects of two different techniques for carpal tunnel injection and their outcomes. Although no significant difference was observed generally between the US-guided and LM-guided groups, longer follow-ups are needed to determine if regression in the outcomes of the LM-guided group is significant or not.
In our clinical context, because of the additional cost of US-guided injection, this procedure is not recommended for corticosteroid injection in CTS based on the results of this study.