1. Background
Iron is a key element in different aspects of life, with a remarkable role in the formation of cell structures. Excessive iron could be potentially toxic as it increments the risk of reactive oxygen species and oxidative stress, which in turn leads to protein, lipid, and nucleic acid oxidation. According to the literature, oxidative stress is the major cause of various pathological reactions (1). Disrupted balance of iron concentrations and oxidative stress, as well as oxidation - reduction activity of iron and reactive oxygen species, may lead to adverse consequences (2, 3).
Iron homeostasis requires regulating mechanisms for the entry of this element into the body and release storage iron for blood formation and iron consumption. Iron affects the tissues through long-term hemodialysis, medical approaches (e.g., stem cell therapy), imaging, tissue engineering, and genetic diseases (anemia, blood transfusions in lethal thalassemia, and hemochromatosis) (4). Furthermore, iron particles are found in food, water, and air pollution (5).
Iron toxicity targets various organs, mainly the liver and kidneys. Liver stores excess iron (6). Iron removal is the therapy of choice for the disorders caused by iron excess, which could save the lives of the patients. Currently, deferoxamine is widely used in the treatment of these disorders, while studies have indicated numerous side-effects (7). Moreover, deferoxamine cannot be absorbed orally and should be administered subcutaneously, which leads to poor patient compliance (8).
New iron-chelating agents have been invented for patients with iron excess-induced disorders, such as thalassaemia. The therapeutic effects of these drugs could be enhanced through appropriate protocols to avoid toxic drug combinations in the patients irresponsive to iron-chelating therapies and those experiencing toxicity complications (9).
Cyanobacteria Arthrospira platensis, also known as Spirulina, is a filamentous, blue-green alga of the Oscillatoriaceae family, which grows abundantly in tropical and subtropical regions. Spirulina has been a major food source for humans, which is rich in proteins, minerals, vitamins (especially B12 family), and various antioxidants (e.g., flavonoids, carotenoid, and phycocyanins) (10, 11). Spirulina has strong associations with iron ions (12) and numerous antioxidant, hypolipemiant, and hepatoprotective properties. Moreover, it protects cells and tissues against aluminum-induced nephrotoxicity (13).
Possible therapeutic effects of Spirulina on the human body were shown with many pre-clinical and clinical studies that suggest several therapeutic effects ranging from reduction of cholesterol and cancer, enhancing the immune system, increasing intestinal lactobacilli, reducing nephrotoxicity by heavy metals and drugs and radiation protection (14).
2. Objectives
The present study aimed to evaluate the protection induced by Spirulina platensis against the toxicity caused by iron in Wistar rats.
3. Methods
3.1. Study Subjects and Design
This randomized, experimental research was conducted on 32 adult, male rats of the Wistar breed (mean weight: 200 ± 20 g). The animals were purchased from the Animal House of Dezful University of Medical Sciences in Dezful, Iran and randomly classified as four groups of eight. The control group (group one) received no treatment. The animals in group two were intraperitoneally administered with iron oxide (15 mg/kg bw/day) as toxicity control (15), group three received oral S. platensis (400 mg/kg bw/day) and iron oxide (15 mg/kg bw/kg) via intraperitoneal injection, and group four was administered with S. platensis only. The treatment continued for 16 days (15).
Adequate food and water were made available to the animals, and they were preserved at room temperature (25 ± 2°C) within a controlled, 12-hour cycle of light and darkness. The experiments were performed based on animal handling safety guidelines.
Iron oxide particles were provided by the Department of Physics at Shahid Chamran University. The powder of S. platensis was purchased from Nanoshimi Yakhteh Company, Iran.
3.2. Sample Preparation and Biochemical Examination
The animals were anesthetized with sodium thiopental (30 mg/kg) 24 hours after administrating the last dosage of the drugs and sacrificed after their weight was measured. A syringe (2 mL) was used to collect blood samples from the hearts of the rats with no anticoagulant. The obtained samples were centrifuged at 3000 rpm for 10 minutes. After separating the serum, the samples were preserved at the temperature of -20°C.
In the following stage, the kidney weight of the animals was measured. In addition, the right kidney and right liver lobes were removed for homogenization, and the concentration of iron was verified in the tissues (16). The left kidney and other lobe of the liver were preserved in 10% formalin saline for histopathology. Afterwards, cross-sections with the diameter of 5 - 6 µ were prepared via paraffin embedding. The obtained samples were stained by Hematoxylin-Eosin for histopathology and histomorphometry. To perform histomorphometry, images of the cross-sections were provided using a Dino-Lite camera equipped with an Olympus optical microscope (magnifications: 4X, 10X, and 40X) at four random points. Data extraction was conducted using the Dino-Lite software.
3.3. Serum Liver and Kidney Function Biomarkers
To assess the animals’ liver function, we measured various parameters, including alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin. Moreover, the levels of AST and ALT were determined based on the substrate enzymatic incubation to generate phenylhydrazine (absorption at 546 nm). The volume of the produced phenylhydrazine was directly proportional to the enzyme quantity (17). At this stage, ALP assay was performed at the transformation point of phenyl phosphate to phenol and phosphate in the enzymatic environment. The released phenol was measured at 520 nm in the presence of 4-aminophenazone and potassium ferricyanide (18).
Total bilirubin was measured based on the reaction between bilirubin in the samples and diazonium salt of sulfanilic acid to produce azobilirubin, which indicated the maximum absorption at 533 nm in the acid medium (19). Investigation of the renal function was based on the levels of serum urea nitrogen (BUN) and creatinine (Cr) using the colorimetric methods proposed by Fawcett and Scott (20) and Peters (21), respectively. Moreover, serum lipid peroxidation and malondialdehyde (MDA) levels were determined. Biochemical markers (e.g., lipid peroxidation) and MDA were also measured using the collected serum samples (22).
3.4. Measurement of Antioxidant Parameters
In the present study, serum thiol protein (GSH) concentration was measured based on the study by Beutler et al., (23). In addition, the generated colored complex was estimated using a spectrophotometer at the wavelength of 450 nm. The chemicals required for this stage of the study were provided by Merck Company (Darmstadt, Germany).
3.5. Serum Total Antioxidant Capacity (TAC)
Ferric-reducing ability of plasma (FRAP) was employed to determine total antioxidant defense (24, 25).
3.6. Statistical Analysis
Data analysis was performed in SPSS version 22 using one-way analysis of variance (ANOVA) to determine the variances and Fisher’s least significant difference test (LSD) test to determine the inter-group differences. In all the statistical analyses, the level of significance was considered to be P ≤ 0.05.
4. Results
Table 1 shows the effects of iron oxide exposure alone and after S. platensis administration on the weight of the body and other organs in the animals of the experimental and control groups. According to the obtained results, body weight significantly reduced in group two (iron toxicity control) (P ≤ 0.05), while the weight of liver increased in this group compared to the controls. However, no significant differences were observed regarding the body and liver weight between the groups receiving S. platensis treatment compared to the controls (P > 0.05). Furthermore, the experiments had no significant effect on the weight of the kidneys.
| Groups | Parameter | ||
|---|---|---|---|
| Body Weight (g) | Liver (g) | Kidney (g) | |
| Group 1 | 238.2 ± 2.6A | 6.28 ± 2.9B | 4.5 ± 1.65A |
| Group 2 | 218.5 ± 2.8A | 8.33 ± 2.4A | 2.9 ± 1.49A |
| Group 3 | 232.7 ± 3.3A | 6.35 ± 4.9B | 4.1 ± 1.52A |
| Group 4 | 236.6 ± 2.9A | 6.77 ± 4.4B | 3.4 ± 1.63A |
aValues are presented as mean ± SE.
bLetters A and B in each column indicate significant differences at P ≤ 0.05.
Table 2 presents the effects of iron oxide exposure alone and S. platensis treatment on the serum biomarkers of liver function, as well as the serum total bilirubin levels and liver marker enzyme activities in the experimental and control groups. According to the findings, iron oxide toxicity caused hepatic damage, which was evident in the significant increase in the levels of bilirubin, AST, ALP, and ALT (P ≤ 0.05). On the other hand, the mentioned biomarkers altered significantly in the groups receiving iron oxide and S. platensis compared to those administered with iron oxide only (P ≤ 0.05). Therefore, it could be concluded that the liver function enhanced in these study groups. However, no significant differences were observed between the animals treated by S. platensis alone compared to the controls in this regard.
| Groups | Parameter | |||
|---|---|---|---|---|
| AST (U.L-1) | ALT (U.L-1) | ALP (U.L-1) | Bilirubin (mg.dL-1) | |
| Group 1 | 161.3 ± 4.2B | 78.5 ± 3.6B | 445.3 ± 3.8B | 2.7 ± 0.32B |
| Group 2 | 367.5 ± 3.7A | 170.7 ± 4.9A | 895.1 ± 5.6A | 4.2 ± 0.94A |
| Group 3 | 170.7 ± 3.3B | 80.2 ± 2.3B | 482.6 ± 3.9B | 4.6 ± 0.34B |
| Group 4 | 155.3 ± 2.2B | 77.8 ± 5.5B | 455 ± 5.1B | 3.7 ± 0.29B |
aValues are presented as mean ± SE.
bLetters A and B in each column indicate significant differences at P ≤ 0.05.
Table 3 shows the effects of exposure to iron oxide alone and S. platensis treatment on the serum biomarkers of renal function. According to the information in this table, urea and creatinine levels increased significantly in the animals exposed to iron oxide compared to the controls (P ≤ 0.05). In addition, renal function biomarkers proved to be significantly normal in the animals receiving treatment by S. platensis (P ≤ 0.05).
| Groups | Parameter | |
|---|---|---|
| Creatinine (mg.dL-1) | Urea (mg.dL-1) | |
| Group 1 | 3.4 ± 0.87B | 26.2 ± 3.4B |
| Group 2 | 4.1 ± 3.11A | 55.4 ± 1.9A |
| Group 3 | 3.7 ± 0.98B | 25.7 ± 1.7B |
| Group 4 | 2.7 ± 0.79B | 27 ± 4.4B |
aValues are presented as mean ± SE.
bLetters A and B in each column indicate significant differences at P ≤ 0.05.
Table 4 shows the impact of iron oxide exposure alone and S. platensis treatment on serum anti-oxidative biomarkers. Accordingly, MDA (lipid peroxidation marker) increased significantly in the animals exposed to iron oxide toxicity compared to the controls (P ≤ 0.05). Furthermore, GSH and TAC significantly declined in the rats exposed to iron oxide compared to the other groups (P ≤ 0.05). Our findings indicated that the mentioned parameters enhanced remarkably in the animals receiving S. platensis treatment with and without iron oxide compared to the controls. However, the effects of S. platensis treatment alone were more evident on these parameters, which could be attributed to the lack of iron oxide toxicity, which in turn had no effect on oxidative balance.
| Groups | Parameter | ||
|---|---|---|---|
| MDA (nmol.mL-1) | G-SH (µmol.mL-1) | TAC | |
| Group 1 | 487.4 ± 3.7B | 52.9 ± 5.5A | 579.8 ± 3.9A |
| Group 2 | 642.8 ± 3.3A | 29.3 ± 4.1B | 429.4 ± 3.1B |
| Group 3 | 498.5 ± 3.7B | 49.8 ± 4.3A | 524.5 ± 2.4A |
| Group 4 | 488.3 ± 6.1A | 55.1 ± 6.4A | 548.2 ± 4.3A |
aValues are presented as mean ± SE.
bLetters A and B in each column indicate significant differences at P ≤ 0.05.
4.1. Iron Oxide Concentration in Liver and Kidneys
After the administration of iron oxide and removal of the liver and kidneys from the animals for the assessment of iron concentrations in the tissues, liver and kidney tissues were investigated separately, and iron measurements were performed using a 700 atomic absorption spectrometer. According to the results, iron concentration increased significantly in the renal and hepatic tissues of the animals administered with iron oxide only compared to the control group (P ≤ 0.05). However, no significant differences were observed between the groups administered with S. platensis with and without iron oxide in terms of iron accumulation in the liver and kidneys (P > 0.05). Iron accumulation in the liver and kidney tissues is presented in Table 5.
| Groups | Parameter | |
|---|---|---|
| Kidneys | Liver | |
| Group 1 | 4.37 ± 4.1A | 6.23 ± 2.4A |
| Group 2 | 11.24 ± 2.8B | 14.8 ± 3.7B |
| Group 3 | 6.52 ± 3.4A | 7.98 ± 4.1A |
| Group 4 | 5.38 ± 4.9A | 6.14 ± 3.3A |
aValues are presented as mean ± SE.
bLetters A and B in each column indicate significant differences at P ≤ 0.05.
4.2. Effects of Histopathological Changes on Studied Organs
4.2.1. Kidneys
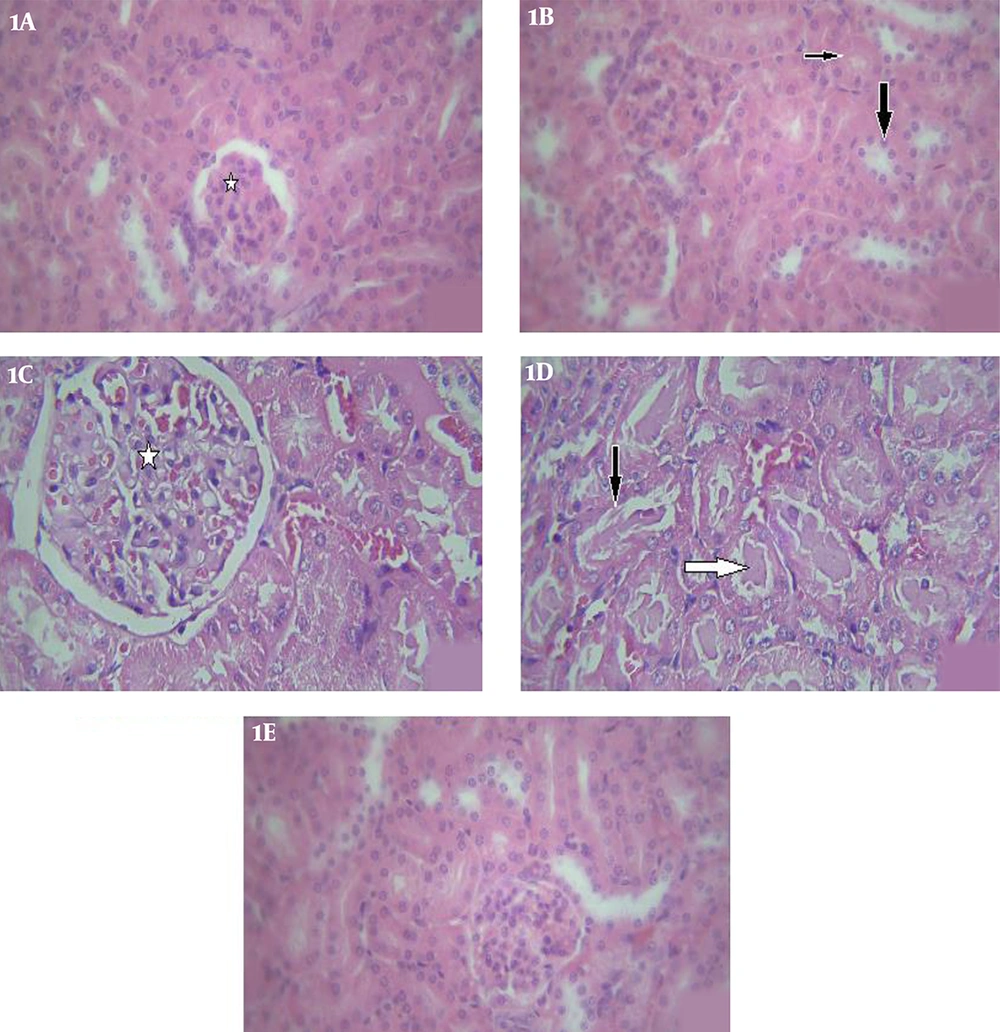
Microscopic examination was performed randomly on three cross-sections obtained from each animal, as well as four fields of each cross-section. According to the results, the structures of the renal cortex and medulla of the kidneys were normal histologically in the normal controls (Figures 1A and 1B). The key indicators of kidney damage were swelling, acute tubular necrosis (ATN), urinary tubule casts, glomerular congestion, and mean outer-to-inner-diameter ratio of the proximal urinary tubules.
Glomerular congestion and swelling of the proximal convoluted tubules were detected in the kidney cross-sections of the animals exposed to iron oxide, as well as maximum casts, which increased significantly compared to the controls (Figures 1C and 1D). Furthermore, ATN was observed in the cross-sections with damaged epithelial cells in these animals. According to the findings, treatment by S. platensis and iron oxide enhanced most of the changes in renal morphology, which was evident in the insignificant glomerular congestion and cell swelling with normal renal tubules. However, no significant difference was observed in the ATN between the experimental and control groups (Figure 1E).
Light photomicrographs of the kidney. 1A, control group: normal renal glomeruli (star) (H&E, X100); 1B, proximal convoluted tubules (thin arrow), distal tubules (thick arrow) (H&E, X100); 1C, Iron oxide treated group: glomerular destruction and congestion (star) (H&E, X400); 1D, iron oxide treated group: showing hyaline casts (black arrow), in some tubules and some tubules with damaged epithelial cells (white arrow) (H&E, X400); 1E, Iron oxide+ S.platensis group: shows normal renal glomeruli and tubules, without any congestion or damaged cells (H&E, X100).
4.2.2. Liver
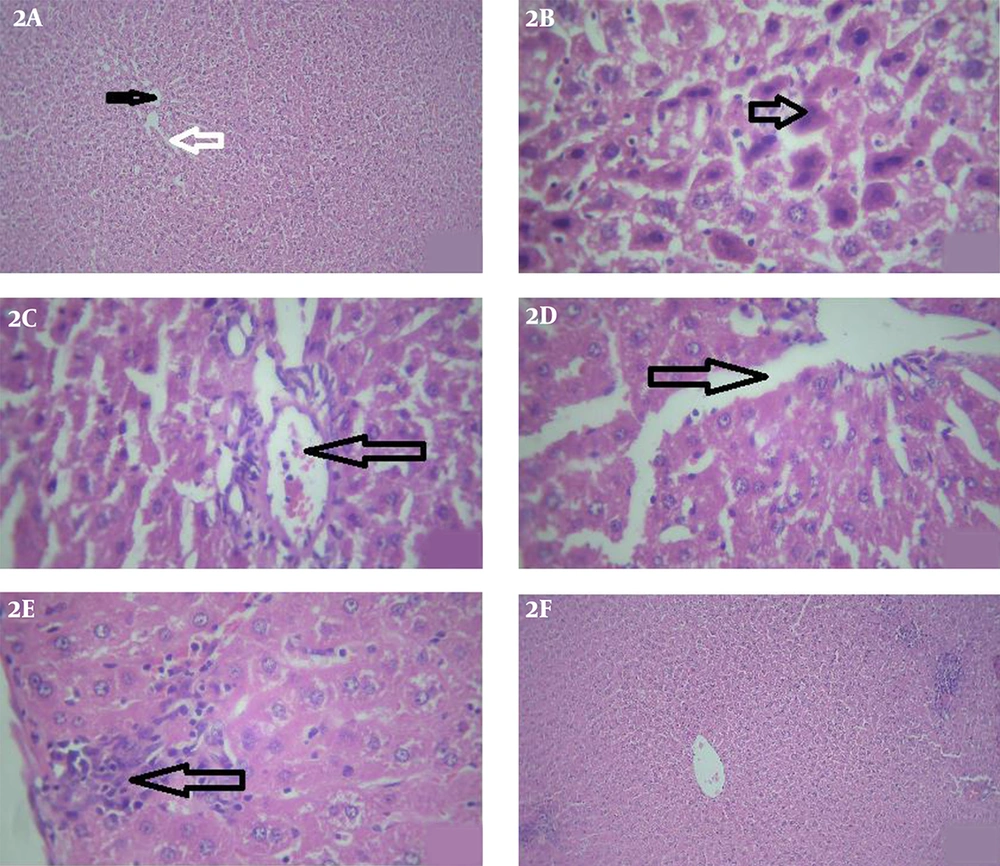
The structure of the liver was normal in the animals of the control group (Figure 2A). According to the histopathological examination of the stained liver cross-sections in the animals exposed to iron oxide, necrotic changes occurred in the hepatocytes (Figure 2B), as well as the congested and dilated central veins (Figure 2C), dilated sinusoids with the higher Kupffer cell proliferation (Figure 2D), lymphocytic infiltration, and regions with mild inflammatory (Figure 2E).
According to the microscopic examination of the liver tissues, hepatocellular changes decreased significantly in the rats receiving S. platensis and iron oxide compared to those exposed to iron oxide only, with similar morphology to that of the animals receiving S. platensis (Figure 2F).
Light photomicrographs of the liver. 2A, control group: shows a central vein (black star) with hepatocyte arranged in cords. Cords of hepatocytes enclose blood sinusoids (white arrow) (H&E, X40). 2B, iron oxide treated group: some hepatocytes show dark nuclei and dense acidophilic cytoplasm (arrow) (H&E, X400). 2C, iron oxide treated group: dilated and congested central veins (arrow) (H&E, X400). 2D, iron oxide treated group: dilated sinusoids (arrow) (H&E, X400). 2E, iron oxide treated group: shows lymphocytic infiltration and mild inflammatory areas (arrows) (H&E, X400). 2F, iron oxide+ S.Platensis group: shows normal central vain without any congestion and ameliorated cords of hepatocytes enclose blood sinusoids (H&E, X40).
5. Discussion
Iron accumulation is often secondary to repeated blood transfusions or caused by excessive gastrointestinal absorption. There are no mechanisms for the excretion of excessive iron in the human body; iron is typically crystallized in the form of iron oxide within ferritin and hemosiderin.
The tissue distribution of iron influences the etiology of iron overload. In thalassaemia, iron excess occurs due to transfusional siderosis or disproportionate iron absorption. Transfusion-induce iron overload leads to iron deposition in the reticuloendothelial system of the spleen, liver, and bone marrow. In the advanced stages, iron could also accumulate in the parenchymal cells of the liver, heart, pancreas, and endocrine organs, which are highly sensitive to its toxicity (26).
Oxidative stress is a pathological state, which is caused by the chemical interactions of the free radicals and damages in the biological molecules. According to the literature, oxidative stress plays a pivotal role in several clinical conditions, such as liver and kidney damage. According to the current research, oxidative stress increased in the Wistar rats with iron overload (15).
In the present study, the serum parameters of kidney function (Cr and BUN) elevated in the iron oxide exposure group; these parameters are considered to be important indicators of kidney damage (27). On the same note, our findings indicated that the serum levels of AST, ALT, ALP, and bilirubin were high in the animals exposed to iron oxide since they were sensitive to the liver damage indices due to the cell leakage and loss of the functional integrity of the liver membrane induced by iron intoxication (28). Furthermore, as a confirmatory histopathological examination of the liver, dilated and congested hepatic sinusoids, as well as several signs of degeneration (e.g., pyknosis, foci of necrosis, and vacuolar degeneration) were detected in these animals. Previous studies have reported similar data regarding liver toxicity, which are consistent with the results of the present study (29).
In the current research, superoxide dismutase, catalase, and GSH tissue levels significantly decreased in the animals of the iron oxide group, followed by an increase in the MDA tissue level. Therefore, the potential toxicity of arsenic could be associated with its inhibitory effects on the antioxidant defense system (GSH and TAC); this finding is in line with the study by Cairo et al., (30). This process occurs through the reactive oxygen species that are generated by iron, which lead to the destabilization of cell membranes through lipid peroxidation and MDA utilization as the basic cell deterioration processes. Therefore, it could be concluded that the reduction of GSH and TAC could accelerate iron predisposition in the liver and kidneys, thereby causing oxidative stress (31), as well as variations in the final weight of the body and liver.
According to the results of the present study, S. platensis and iron oxide administration increased serum GSH and TAC compared to treatment with iron oxide alone. Moreover, it decreased serum MDA production, which could be due to the antioxidant effects of S. platensis. This herb has attracted the attention of many researchers due to its active ingredients and considerable phycocyanin, β-carotene (an orange pigment that enhances the antioxidant status), tocopherol, selenium, and phenolic compounds, which are known to have operative antioxidant and anti-inflammatory effects. For instance, phycocyanin is a major water-soluble antioxidant found in S. platensis, which has proven 20 times more effective than vitamin C (32). The active constituents of S. platensis are able to act synergistically to exert intense antioxidant effects. According to the present study, Spirulina could excrete iron from the liver, which is in congruence with the results obtained by Bermejo et al., (33, 34) and Gad et al., (35). Spirulina therapy has also been shown to be chelating and excrete iron from the body through effective chelation. Another suggested mechanism for the reduction of iron after Spirulina therapy is the high content of metallothioneins, which are the proteins able to bind to heavy metals and excrete them from the body (33). Whether administered alone (to assess the possible side-effects) or combined with iron oxide in the animals in the current research, S. platensis had no significant difference with the control group; this finding is inconsistent with the study by Bashandy et al., (13). Also some researchers conclude that aqueous S. platensis extracts contain antiretroviral activity that may be of potential clinical interest (36).
5.1. Conclusions
According to the results, S. platensis exerts protective effects on the rats receiving iron treatment through preventing oxidative stress damages to the liver and kidneys. In addition, S. platensis could restore the normal antioxidant status in these rats. S. platensis could also inhibit the pathological changes induced by iron oxide treatment in the liver and kidneys. It is recommended that further investigation in this regard be focused on the molecular mechanisms involved in the protective functions of S. platensis against iron toxicity.