1. Background
Morbidity and mortality associated with being obese have been known since ancient times (1, 2). Greater body mass index showed to be associated with an increased rate of all causes and cardiovascular death. In addition to mortality, being obese and overweight are associated with increased morbidity resulted from a higher relative risk of hypertension, hypercholesterolemia, diabetes mellitus, stroke, colon cancer, gall stone, osteoarthritis, infertility, sleep apnea, and many others (3).
Weight reduction showed to have multiple health benefits, including a reduction in the rate of progression to diabetes in patients with impaired glucose tolerance, blood pressure in hypertensive patients, and lipid levels in patients with hyperlipidemia. Other reported benefits are reductions in sleep apnea, urinary incontinence, and depression resulting in improvements in quality of life (4). A wide range of dietary, exercise, pharmacologic, and surgical therapies is investigated for their weight reduction effect (5). Dietary supplements are among the most popular interventions for this purpose (6).
Sumac (Rhus coriaria L.) is a popular spice in the Eastern Mediterranean Region. It is from the Anacardiaceae family and grows in different regions such as the Mediterranean, North African countries, as well as Middle Eastern countries (7). More than 180 bioactive compounds (tannins, phenols, flavonoids, terpenoids, etc.) are found in sumac fruits (8). Multiple animal and human studies have demonstrated its potential positive effect on cardiovascular risk factors, including diabetes mellitus (9-11), hyperlipidemia (12-16), insulin resistance, and C-reactive protein (17). However, its effect on obesity, as a major cardiovascular risk factor, is not previously studied.
2. Objectives
This study is aimed at evaluating the efficacy and safety of Rhus coriaria L. (Sumac) supplementation in patients with overweight or obesity in a double-blind randomized controlled trial.
3. Methods
3.1. Trial Design
The trial was planned as a parallel, two-arm, double-blind, randomized placebo-controlled clinical trial with no modifications in methods after trial commencement.
3.2. Participants
One hundred twenty-six patients attending the endocrinology clinic affiliated by Shiraz University of Medical Sciences from May 2016 to September 2016, aged between 20 - 65 years, and with a body mass index (BMI) of more than 25 kg/m2 were evaluated for inclusion in the study. Subjects with hypothyroidism or hyperthyroidism, diabetes mellitus, liver cirrhosis, renal failure, and heart failure were excluded from our study. Those taking anti-hyperglycemic and lipid-lowering drugs, diuretics, and contraceptives were excluded. In addition, a history of allergic reaction to Sumac, alcohol consumption, pregnancy, and lactation were also a part of the exclusion criteria. After all, 50 patients were assigned to the Sumac and placebo groups.
3.3. Sample Size
The sample size was calculated considering the expected efficacy of 1 kg/m2 (18), one-sided significance of 0.05, and power of 0.80 for the investigational design. The required sample size worked out to be 20 participants per group. Considering some level of 20% risk for subjects’ dropout during the study, an additional group of five more volunteers were added onto each group in the investigation.
3.4. Drug and Placebo Preparation
Rhus coriaria L. (Sumac) fruit was provided from local marketplaces in the Shiraz (Southern Iran). A botanist at the School of Pharmacy, Shiraz University of Medical Sciences identified the sample. A voucher specimen with the number of PM 533 was deposited in the Shiraz School of Pharmacy herbarium. The sumac fruit was powdered and sieved by Mesh 60. Each capsule was loaded with 500 mg of Sumac powder or placebo. Quality control tests, including weight control test for capsules and microbial contamination, were done based on guidelines of The United States pharmacopeia. White wheat flour was applied as the placebo.
3.5. Interventions
Participants were instructed to take 500 mg capsules containing Sumac or placebo two times per day after their meals for six weeks (based on the mean dose used in previous studies) (19). All patients were also recommended to maintain a routine aerobic physical activity at least five times per week, each time for 30 minutes. They were also instructed to go on a low-lipid, low-carbohydrate, and low-salt diet. The patients were recommended to take a note from their compliance to their drug and life style recommendations in a specific form. Finally, they were instructed to report any side effect to the physician via a specific phone number.
3.6. Outcomes
All patients were assessed before, and six weeks following the intervention in terms of body measures including weight, height, BMI, hip circumference (HC), waist circumference (WC), and waist-hip ratio (WHR). Serum samples for determination of levels of fasting glucose, lipids, insulin, and leptin were also collected before and after the study. All the samples were collected after eight hours of fasting in the morning and frozen to be analyzed at the end of study all together. Safety outcomes included liver and kidney function tests, and any other subjective adverse event reports.
3.7. Randomization and Blinding
Fifty eligible patients were enrolled in the study by the main clinical physician (3rd author) and randomly assigned to the groups by the clinic secretary who had been trained on application of the randomization list, which was created using the block randomization method by the Microsoft Excel software (version 2007, Microsoft Corporation, USA). The randomization list was created by principle investigator (last author). The physicians and researchers all remained blind to the assignment of patients. According to the similar shape and size of capsules and containers of Sumac and placebo, the patients were also blind to the intervention allocation.
3.8. Statistical Analysis
Statistical Package for the Social Sciences Statistics software (version 23) was applied for data analysis. The descriptive data were presented by means, standard deviations, numbers, and percentage in case of quantitative and qualitative data, respectively. Mann-Whitney U test, Wilcoxon signed-rank test, and chi-square test were applied for statistical comparison of basic and final values within and between Sumac and placebo groups. A P value of less than 0.05 was deemed significant.
3.9. Ethical Considerations
The study protocol was approved by the Local Medical Ethics Committee of Shiraz University of Medical Sciences to be in compliance with the Declaration of Helsinki (ethical approval number: CT-92-5581, IRB code: CT-92-5581). All patients were informed in detail about the study protocol and signed a written informed consent for participation in the research. The study predetermined method was registered in the Clinical Trials Registry (registration ID: NCT02754089).
4. Results
4.1. Study Flow and Baseline Characteristics
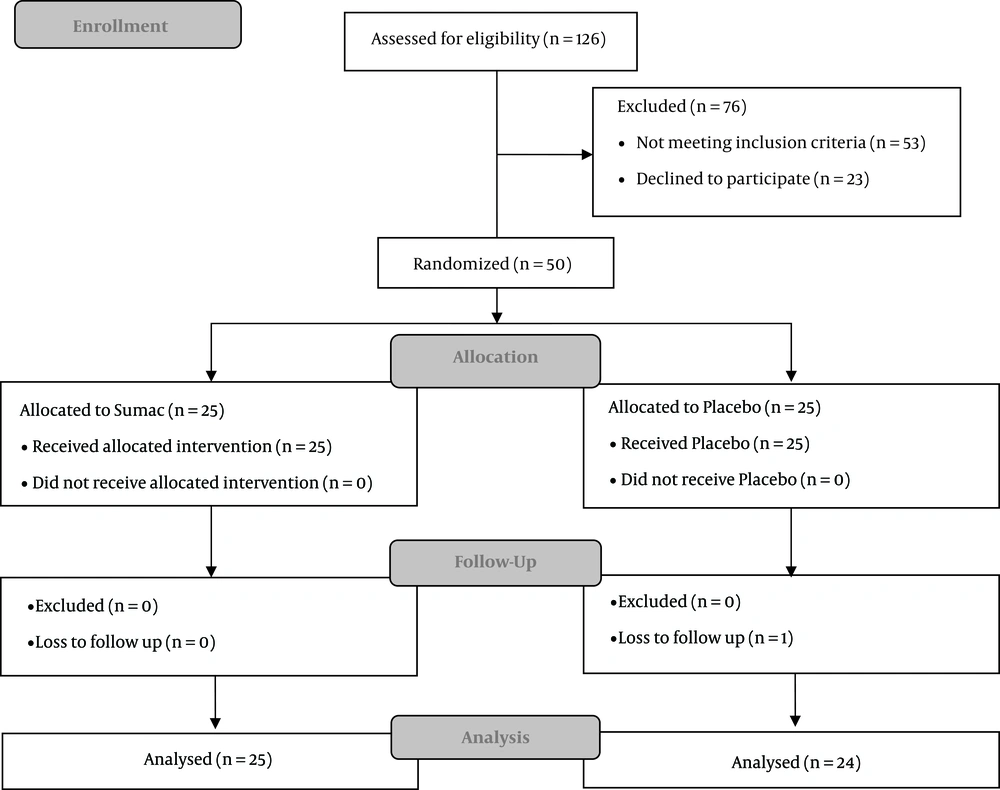
From May 2016 to September 2016, a total of 126 participants were assessed for eligibility and, finally, 50 of them were randomly assigned to receive either Sumac supplementation or placebo (25 participants in each group). One patient was lost to be followed-up in the placebo group due to not responding to contacts. At last, 49 patients were brought into the analysis. Figure 1 is the flowchart revealing detailed descriptions of patients' enrolment, randomization, and outcomes. No significant difference was observed between study groups regarding their demographic and clinical baseline characteristics (Table 1).
| Characteristics | Sumac | Placebo | P Value |
|---|---|---|---|
| Age, y | 45.16 ± 5.48 | 43.13 ± 9.22 | 0.346b |
| Gender, male/female | 14/11 | 14/10 | 0.869c |
| Smoking, yes/no | 5/20 | 8/16 | 0.291c |
| Weight, kg | 81.80 ± 10.23 | 81.40 ± 11.71 | 0.968b |
| Height, m | 1.65 ± 0.09 | 1.64 ± 0.08 | 0.741b |
| BMI, kg/m2 | 30.20 ± 3.81 | 30.08 ± 3.10 | 0.920b |
| Fasting blood glucose, mg/dL | 83.29 ± 6.73 | 83.40 ± 10.26 | 0.818b |
| Insulin, mU/L | 13.98 ± 3.79 | 18.30 ± 6.65 | 0.120b |
| Insulin resistance index (HOMA) | 2.89 ± .88 | 3.71 ± 1.24 | 0.088b |
| Leptin, ng/mL | 18.40 ± 17.02 | 8.53 ± 7.09 | 0.014b |
aValues are expressed as mean ± SD.
bMann-Whitney U test.
cChi square test.
4.2. Outcome Measures
Statistically significant decrease in weight, waist circumference, and BMI were observed in the Sumac group, which was more prominent as compared to the placebo after six weeks of intervention (P = 0.003, P = 0.027, and P = 0.002, respectively) (Table 2). Insulin resistance was also decreased (P = 0.020). No significant change in leptin and fasting blood glucose was observed. No clinical or laboratory adverse event was observed (Table 3).
| Variables | Intervention Group | P Valueb | |
|---|---|---|---|
| Sumac (N = 25) | Placebo (N = 24) | ||
| Weight, kg | 0.003 | ||
| Before | 81.80 ± 10.23 | 81.40 ± 11.71 | |
| After | 80.46 ± 10.37 | 80.75 ± 11.35 | |
| Difference | 1.34 ± 0.74 | 0.64 ± 0.92 | |
| P valuec | < 0.001 | 0.002 | |
| Waist circumference, cm | 0.027 | ||
| Before | 103.48 ± 7.67 | 105.29 ± 9.34 | |
| After | 102.38 ± 7.73 | 104.75 ± 8.95 | |
| Difference | 1.10 ± 0.72 | 0.54 ± 1.11 | |
| P valuec | < 0.001 | 0.026 | |
| Hip circumference, cm | 0.061 | ||
| Before | 111.52 ± 9.51 | 109.67 ± 7.63 | |
| After | 110.82 ± 9.52 | 109.35 ± 7.52 | |
| Difference | 0.70 ± 1.02 | 0.31 ± 0.76 | |
| P valuec | 0.002 | 0.057 | |
| Waist-hip ratio | 0.063 | ||
| Before | 0.929 ± 0.040 | 0.962 ± 0.076 | |
| After | 0.925 ± 0.041 | 0.960 ± 0076 | |
| Difference | 0.004 ± 0.00 | 0.002 ± 0.00 | |
| P valuec | 0.006 | 0.220 | |
| BMI, kg/m2 | 0.002 | ||
| Before | 30.20 ± 3.81 | 30.08 ± 3.10 | |
| After | 29.71 ± 3.83 | 29.83 ± 2.91 | |
| Difference | 0.49 ± 0.27 | 0.24 ± 0.35 | |
| P valuec | < 0.001 | 0.003 | |
aValues are expressed as mean ± SD.
bBetween group analysis by Mann-Whitney U test (comparing mean reductions between two groups).
cWithin group analysis by paired t test.
| Variables | Intervention Group | P Valueb | |
|---|---|---|---|
| Sumac (N = 34) | Placebo (N = 36) | ||
| Fasting blood glucose, mg/dL | 0.382 | ||
| Before | 83.40 ± 10.26 | 83.29 ± 6.73 | |
| After | 80.56 ± 10.50 | 82.00 ± 7.17 | |
| Difference | 2.84 ± 8.40 | 1.29 ± 7.76 | |
| P valuec | 0.104 | 0.423 | |
| Insulin, mU/L | 0.012 | ||
| Before | 18.30 ± 6.65 | 13.98 ± 3.79 | |
| After | 16.44 ± 6.18 | 15.00 ± 6.73 | |
| Difference | 1.86 ± 5.73 | -1.01 ± 5.15 | |
| P valuec | 0.117 | 0.344 | |
| Insulin resistance index (HOMA) | 0.020 | ||
| Before | 3.71 ± 1.24 | 2.89 ± 0.88 | |
| After | 3.25 ± 1.24 | 3.04 ± 1.35 | |
| Difference | 0.45 ± 1.29 | -0.14 ± 0.95 | |
| P valuec | 0.090 | 0.473 | |
| Leptin, ng/mL | 0.334 | ||
| Before | 8.53 ± 7.09 | 18.40 ± 17.02 | |
| After | 9.30 ± 7.64 | 20.18 ± 17.49 | |
| Difference | -0.76 ± 3.42 | -1.77 ± 9.05 | |
| P valuec | 0.273 | 0.346 | |
aValues are expressed as mean ± SD.
bBetween group analysis by Mann-Whitney U test (comparing mean reductions between two groups).
cWithin group analysis by paired t-test.
The analysis of covariates showed that the change in weight of patients in the Sumac group was not associated with changes in the leptin level. The reduction in insulin resistance was also independent from the effect of Sumac on weight reduction.
4.3. Safety Measures
No clinical or laboratory adverse event was observed in study patients.
5. Discussion
The current study indicated that the supplementation of 1000 mg/day of Sumac dried fruit powder in patients with obesity or overweight (BMI > 25 kg/m2) would demonstrate a statistically significant decrease in their weight, BMI, and waist circumference. Reduction in the insulin resistance of these patients was another observed benefit of Sumac supplementation in these patients.
Rhus coriaria is a popular medicinal plant in Middle Eastern countries. It is mentioned by traditional Persian scholars such as Rhazes (865 - 925 CE) (20), Avicenna (980 - 1037 CE) (21), Haly Abbas (930 - 994 CE) (22), Jorjani (1137 CE) (23), and Aghili Shirazi (1670 - 1747 AD) (24) as a treatment for obesity.
Multiple studies have evaluated the efficacy of Rhus coriaria on metabolic disorders including diabetes and hyperlipidemia. Methanolic extract of Rhus coriaria showed to decrease triglyceride and cholesterol in high cholesterol diet-fed rats (15). Two other studies have confirmed beneficial effects of Rhus coriaria in hyperlipidemia in clinical settings (14, 16). Rhus coriaria supplementation in patients with diabetes mellitus is also investigated in two clinical trials (11). Rahideh et al. showed improvement in insulin resistance, oxidative stress, and chronic inflammatory indices of patients with diabetes by three month supplementation with 3 gr/day dose of Rhus coriaria. Shidfar et al. also presented hypoglycemic effect of Rhus coriaria beside improvement in APO A-1 and APO-B in Diabetic patients.
Based on our results, the observed effect of Rhus coriaria against obesity in our study was not through the change in leptin associated pathways. However, the aqueous extracts of Rhus coriaria showed to have a significant anti lipase effect with IC50 values 19.95 mcg/mL in previous studies, which can suggest a potential anti-obesity mechanism of Rhus coriaria (25, 26).
The most important limitations of this study were limited sample size and also the short duration of the intervention, which affect the generalizability of the results. Lack of standardization of the product is another important limitation of the study. Despite the similar recommendation on diet and physical activity in both groups, lack of standard measurement on these confounding variables cause uncertainty about the patients’ similar compliance to these recommendations in both groups. Further studies covering these limitations are needed to more precisely evaluate Sumac efficaciousness in obesity. In conclusion, the study showed anti-obesity and insulin sensitizing effect of Rhus coriaria supplementation in patients who are overweight and obese. This effect was a clinically small size, however, it was statistically significant.