1. Background
In recent decades, there has been an increasing trend in diabetes prevalence in all countries worldwide (1). Based on the global report on diabetes by WHO, 108 million people had diabetes in 1980 worldwide while this number increased to 442 million by 2014 (2). In this period (1980 to 2014), diabetes prevalence in the adult population increased from 4.7% to 8.5% (2, 3). The burden of the disease including considerable premature deaths, disabilities, and costs has made diabetes one of the most important chronic diseases for public health (3, 4).
Diabetes is associated with both microvascular and macrovascular complications such as ischemic heart disease, cerebrovascular accident, nephropathy, retinopathy, and neuropathy (3, 5). Diabetes Foot Ulcer (DFU) is one of the most important complications of the disease that occurs in 15% - 25% of diabetic patients during their lifetime (6, 7). The annual incidence rate of DFU has been estimated at 2% - 4% (7, 8). The DFU is a common, serious, and preventable complication of diabetes, accounting for the cause of over 50% of lower limb amputation. Nearly, one-third of the cost of diabetes is due to this complication (7, 8). The DFU is a major cause of hospitalization in diabetic patients, which could lead to infection, foot amputation, decreased quality of life, and premature death (7, 9, 10). A study in Iran showed that the cumulative incidence of DFU was 5.6% in a two-year period (8). Moreover, it has been estimated that the financial burden of diabetes complications in the country is one billion USD, of which 107 million (10.7%) is due to DFU (4).
Various factors including sociodemographic characteristics, diabetes management-related factors, and behavioral factors such as smoking have been reported as the most important determining factors of DFU (11-14).
2. Objectives
This study aimed to assess the DFU prevalence and its related factors among diabetic patients in the Diabetes Center of Kerman University of Medical Sciences.
3. Methods
A cross-sectional study was performed from January to March 2017. The study population consisted of adult diabetic patients in the Diabetes Center of Kerman University of Medical Sciences. In the healthcare system of Iran, diabetes centers with multidisciplinary teams provide comprehensive care for diabetic patients as the third level of healthcare service delivery. A total of 400 patients were enrolled in the study using a convenience sampling method. The inclusion criteria were an age of over 18 years or older, having type 2 diabetes mellitus, and taking diabetes-related healthcare for at least one year in the Diabetes Center.
The required data were collected using a specific questionnaire. The first part of this instrument included demographic data (age, sex, marital status, education level, job, and income) and data related to the disease (disease duration, types of medications for glycemic control, diabetes-related complications, number of blood glucose checks, number of medical visits to a physician in the previous year, and Glycosylated hemoglobin A1C (HbA1c) level).
In the second part of the form, we assessed the DFU occurrence during the disease course and the previous year, foot amputation, and hospital admission due to DFU. We defined DFU as a full-thickness wound that existed at a level distal to the ankle in patients with diabetes (9, 10). Data were collected using patients’ medical records, interviews with the participants, and physical examination.
The study objectives and data confidentiality were explained to each participant and written consent was obtained. Furthermore, the study proposal was approved by the Ethics Committee of Kerman University of Medical Sciences (ethical code: IR.KMU.AH.REC.1395.74). The collected data were imported to SPSS version 22 software. The descriptive data were presented by frequency, percentage, mean, standard deviation, and tables. The chi-square (χ2) test and independent t-test were also employed for comparing the differences between subgroups. Binary logistic regression analysis was performed to determine the predicting factors of DFU. The independent variables with a P value of lower than 0.2 in univariate analysis were entered into the regression model. An alpha value of equal to 0.05 was considered as the significance level.
4. Results
Of 400 diabetic patients enrolled in the study, 69% (n = 276) were female and 73.2% (n = 293) were married. The mean (± SD) of their age was 57.1 (± 11.9) years and 72% (n = 288) were younger than 65. The education level was high school or less in 77.3% (n = 307); 47% (n = 186) were homemakers and 31.7% (± 125) reported their household income was quite appropriate to meet their living costs (Table 1). The mean (± SD) of disease duration in the participants was 11.2 (± 7.7) years and it was 10 years or less in 49.7% (n = 199) of the patients. About 59% (n = 236) of the patients had insulin alone or in combination with oral hypoglycemic drugs in their medication regimen. Also, 59% (n = 235) of them had at least one microvascular or macrovascular diabetes complications and more than one-third (n = 137, 34.2%) of them were current smokers. Of the total subjects, 74.5% (n = 298) were educated regarding foot care. The mean number of visits to a physician for taking diabetes care and the frequency of blood glucose checks in the previous year were 3.7 (SD = 3.3, median = 3) and 4.8 (SD = 2.9, median = 6), respectively. The mean (± SD) of the A1C hemoglobin level was 13.2 (2.6) mg/dL.
| Variables/Categories | No. (%) | Diabetic Foot Ulcer | ||
|---|---|---|---|---|
| Yes, No. (%) | No, No. (%) | P Value | ||
| Age groups, y | < 0.001 | |||
| < 50 | 112 (28.0) | 7 (6.2) | 105 (93.8) | |
| 50 - 64 | 176 (44.0) | 33 (18.8) | 143 (81.2) | |
| > 64 | 112 (28.0) | 29 (25.9) | 83 (74.1) | |
| Gender | 0.042 | |||
| Female | 276 (69.0) | 41 (14.9) | 235 (85.1) | |
| Male | 124 (31.0) | 28 (22.6) | 96 (77.4) | |
| Marital status | 0.268 | |||
| Married | 293 (73.2) | 48 (16.4) | 245 (83.6) | |
| Unmarried | 107 (26.8) | 21 (19.6) | 86 (80.3) | |
| Education level | 0.144 | |||
| Secondary or less | 140 (35.0) | 22 (15.7) | 118 (84.3) | |
| High school | 169 (42.3) | 36 (21.3) | 133 (78.7) | |
| Academic | 91 (22.7) | 11 (12.1) | 80 (87.9) | |
| Job | 0.688 | |||
| Housewife | 188 (47.0) | 28 (14.9) | 160 (85.1) | |
| Employed | 97 (24.3) | 19 (19.6) | 78 (80.4) | |
| Retired | 71 (18.7) | 13 (18.3) | 58 (81.7) | |
| Unemployed | 44 (11.0) | 9 (20.5) | 35 (79.5) | |
| Household income | 0.546 | |||
| Quite appropriate | 126 (31.5) | 18 (14.3) | 108 (85.7) | |
| Relatively appropriate | 200 (50.0) | 38 (19.0) | 162 (81.0) | |
| Quite inappropriate | 74 (18.5) | 13 (17.6) | 61 (82.4) | |
| Smoking | < 0.001 | |||
| Yes | 137 (34.2) | 42 (30.7) | 95 (69.3) | |
| No | 263 (65.8) | 27 (10.3) | 236 (89.7) | |
| Disease duration, y | 0.001 | |||
| < 10 | 201 (50.3) | 23 (11.4) | 178 (88.6) | |
| ≥ 10 | 199 (49.7) | 46 (23.1) | 153 (76.9) | |
| Type of medication | 0.004 | |||
| Oral hypoglycemic agents | 164 (41.0) | 18 (11.0) | 146 (89.0) | |
| Insulin | 236 (59.0) | 51 (21.6) | 185 (78.4) | |
| Diabetes-related complications | < 0.001 | |||
| Yes | 235 (58.8) | 61 (26.0) | 174 (74.0) | |
| No | 165 (41.2) | 8 (4.8) | 157 (95.2) | |
| Number of visits to physicians in the previous year | 0.113 | |||
| < 3 | 168 (42.0) | 34 (20.2) | 134 (79.8) | |
| ≥ 3 | 232 (58.0) | 35 (15.1) | 179 (84.9) | |
| Blood glucose checks, No./y | 0.090 | |||
| < 6 | 188 (47.0) | 38 (20.2) | 150 (79.8) | |
| ≥ 6 | 212 (53.0) | 31 (14.6) | 222 (85.4) | |
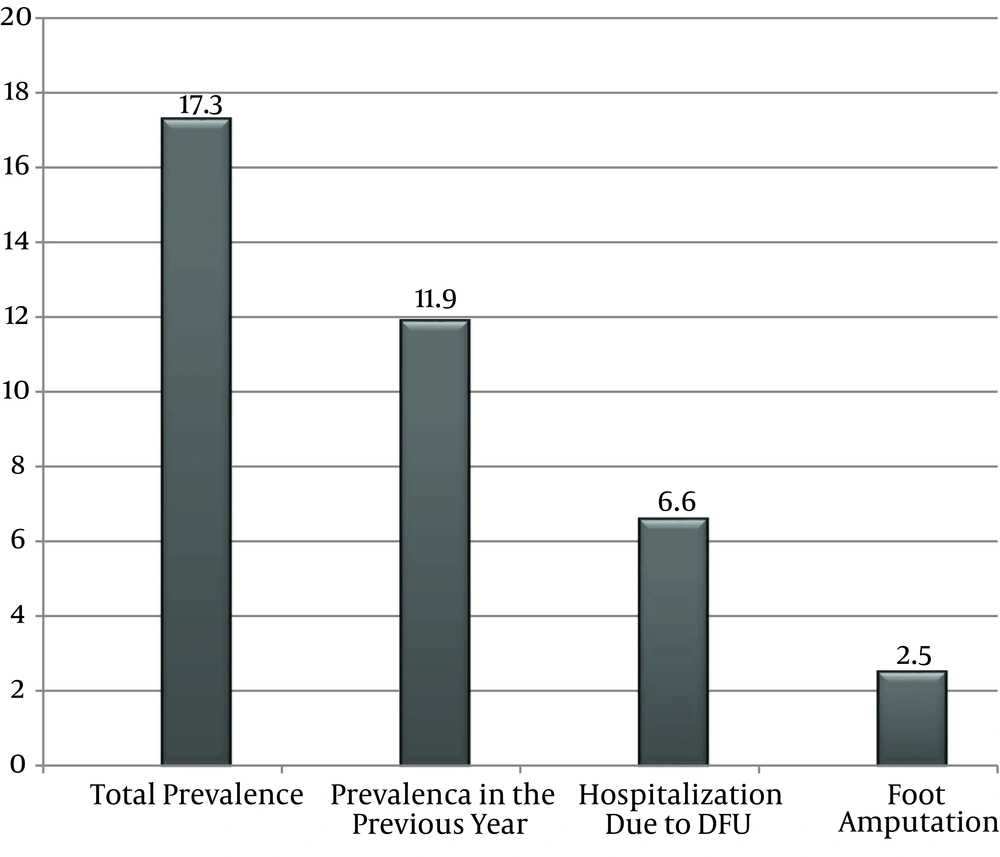
Diabetic foot ulcer frequency was 17.3% (n = 69) among diabetic patients during the total course of their disease (history of DFU during disease period). Moreover, 47 subjects had DFU in the previous year that represented an annual prevalence of 11.8%. Of the 47 DFU cases, 16.7% (n = 8) were diagnosed as new cases that did not have any past history of DFU and 83.3% (n = 39) had a history of at least one DFU. Besides, 27 of the total participants (6.6% of all patients and 37.7% of DFU patients) were admitted to a hospital due to DFU. Major or minor amputation was performed for 14.5% (n = 10) of the total DFU cases and 2.5% of the total patients (Figure 1).
There was a statistically significant relationship between DFU frequency and age (P < 0.001). The highest frequency was in patients older than 64 years (25.9%), followed by the age groups of 50 - 64 years (18.8%) and less than 50 years (6.2%). The male patients had a higher DFU frequency than female patients (22.6% vs. 14.9%; P = 0.042). The DFU frequency did not show statistically significant relationships with marital status (P = 0.268), education level (P = 0.144), job category (P = 0.688), and household income (P = 0.546) (Table 1). The DFU frequency was 30.7% in smokers, which was significantly higher than in non-smokers (10.3%) (P < 0.001). Patients with disease duration of more than 10 years (23.1%) had a higher DFU frequency than those with disease duration of 10 years or less (11.4%) (P = 0.001).
The DFU frequency had a significant relationship with the type of medication (P = 0.013). The frequency was higher in patients having insulin in their drug regimen (21.6%) than in those who took oral hypoglycemic drugs (11.0%). Also, the DFU frequency was higher in patients with diabetes-related complications than in those without complications (26.0% vs. 4.8%, P < 0.001). The DFU frequency had no relationship with the frequency of blood glucose checks (P = 0.090) and the frequency of visits to a physician in the previous year (P = 0.113). The mean A1C level did not show any significant difference between patients with DFU (mean = 13.35, SD = 2.74) and patients without DFU (mean = 13.15, SD = 2.09) (P = 0.581).
The results of logistic regression analysis for determining the predicting factors of DFU in diabetic patients showed that three variables including age group, smoking, and diabetes-related complications were significant in the multivariable model. Having diabetes-related complications was the strongest predictor of DFU (OR = 7.016, 95% CI = 2.67 - 18.38) such that the DFU risk was over seven times greater in patients having micro-vascular or macro-vascular complications. In the next rank, being a smoker (OR = 3.80, 95% CI = 2.06 - 6.99) increased the risk approximately four times. Also, the risk of DFU was 3.7 times more in patients aged over 64 years than in those younger than 50 years (OR = 3.70, 95% CI = 1.299 - 10.568) (Table 2).
| Predictors | B | SE | Exp(B) | P Value | 95% Confidence Interval for Exp (B) |
|---|---|---|---|---|---|
| Constant | -4.690 | 0.630 | 0.009 | 0.000 | |
| Age | |||||
| < 50 | Reference | ||||
| 50 - 64 | 0.843 | 0.527 | 2.323 | 0.110 | 0.827 - 6.523 |
| > 64 | 1.310 | 0.535 | 3.705 | 0.014 | 1.299 - 10.568 |
| Smoking | 1.336 | 0.311 | 3.802 | 0.000 | 2.068 - 6.990 |
| Diabetes-related complications | 1.948 | 0.491 | 7.016 | 0.000 | 2.677 - 18.383 |
5. Discussion
The results of the current study revealed that 17.3% of diabetic patients had DFU during the disease course and the annual prevalence of DFU was 11.8%. A meta-analysis in 2017 showed that the DFU prevalence is 6.3% in the world and 5.5% in Asia (7). A systematic review in six Arab countries showed that the mean prevalence of DFU was 6% (15). Thus, the frequency of DFU in the current study was higher than the DFU prevalence in the world, Asia, and the Arab world (7, 15). However, some studies in line with our study had reported a higher frequency of DFU. For instance, studies in Sudan, India, and North America have reported the DFU prevalence of 18.1%, 14.3%, and 13.0%, respectively (7, 11, 12). As one of the most common diabetes-related complications, DFU leads to serious outcomes such as premature mortality, disability, and low quality of life (16). Therefore, in diabetes care, DFU should be considered an important health problem and patients should be trained for foot care practice.
Based on our results, 6.6% of the total patients and 37.7% of patients with DFU had a history of hospitalization due to DFU. Also, 2.5% of the total participants and 14.5% of patients with DFU had foot amputation. The DFU is a leading cause of hospital admission in diabetic patients (9). The studies found that between 20 and 50% of hospitalization episodes of diabetic patients were due to DFU (9, 17). A study showed that the mean duration of hospitalization was 11.4 days and the cost imposed on each hospitalized DFU patient was 7636 Euros (18). Therefore, patient education and performing DFU care by health caregivers can decrease the burden of DFU and prevent hospitalization and foot amputation.
The results of the study showed that the DFU prevalence was higher in older age groups such that the risk of DFU was 3.7 times more in patients aged over 64 years than in patients younger than 50 years. Consistent with this finding, several studies have reported a higher prevalence of DFU in older age groups (7, 12, 14). Older diabetic patients mostly show a long duration of the disease associated with various diabetes-related complications and other chronic diseases that could explain the higher risk of DFU in older age groups (6, 19, 20).
Male patients had significantly higher DFU prevalence than female patients (22.9% vs. 14.6%). A higher DFU frequency was reported in male diabetic patients in several studies (7, 12, 20). Some studies have shown that men are at greater risk of DFU due to having a higher rate of risk factors leading to DFU such as smoking, peripheral vascular diseases, limited joint mobility, poor glycemic control, and poor medication adherence (7, 13, 14). Thus, it seems that the higher prevalence of unhealthy behaviors is the main cause of sex-related differences.
Based on the findings of the present study, patients with disease duration of longer than 10 years and those using insulin in their ant-diabetic regimen had higher DFU frequency. Several studies, consistent with these findings, reported longer disease duration and using insulin encounter as important factors associated with DFU occurrence (7, 8, 21, 22). One explanation for this result might be that patients with longer disease duration and insulin users usually are in a more severe and advanced stage of diabetes, which both increases the risk of DFU and other diabetes complications (7, 9, 12, 14, 23). Neuropathy and peripheral vascular diseases as important pathologic contributing factors to DFU are common complications in patients with poor glycemic control, older age, and longer disease duration (24).
This study showed that smoking patients had a higher DFU frequency than non-smoking patients (30.7% vs. 10.3%) and the odds of DFU occurrence were 3.8 times higher in smoking diabetic patients than in non-smoking patients. It is well established that serious factors play an important role in developing DFU, such as decreased blood flow in extremities, peripheral angiopathy, and peripheral neuropathy, that are more common in smokers (13, 25, 26). Furthermore, some studies have reported that smoking aggravates glucose hemostasis and accelerates the onset and progress of microvascular and microvascular diabetes complications (27, 28).
The result of this study showed that the risk of DFU was over seven times greater in diabetic patients with microvascular or macrovascular complications than in those without complications. A large retrospective cohort study revealed that peripheral vascular disease, neuropathy, retinopathy, nephropathy, cerebral vascular disease, and coronary artery disease were the important risk factors of DFU with odds ratios of 8.3, 15.6, 3.9, 3.5, 2.1, and 1.93, respectively (24). Also, Yun et al. reported that DFU was significantly higher in patients with cardiovascular autonomic dysfunction, retinopathy, and nephropathy complications (29). The results of a study in Korea showed that 90% of diabetic patients with DFU had diabetic retinopathy while another study in the US revealed that moderate or severe nephropathy was associated with the increased risk of DFU (30, 31). Microvascular impairment with limited tissue blood supply, macrovascular disorders (such as atherosclerosis and peripheral vascular disease), and neuropathy are the main etiologic factors of DFU (32, 33). One explanation for the association between DFU and other complications can be that they have the same causative factors.
This study had some limitations. First, it was a cross-sectional study and thus, we could not determine the temporal relationship between independent variables and DFU occurrence as the outcome variable. Second, this study was conducted in a clinic at the tertiary level of the Iranian health care system and thus, its results could not be generalized to all diabetic patients. Finally, although neuropathy is an important factor for the development of DFU, the current study did not assess neuropathy and its relationship with DFU.
5.1. Conclusions
The results of the present study demonstrated that the frequency of DFU was high among diabetic patients attending the Diabetes Center of Kerman University of Medical Sciences. Also, hospital admission and foot amputation due to DFU had a considerable frequency. Therefore, there is a crucial need to improve the quality of foot care services. Furthermore, to decrease the risk of DFU, it is necessary to highlight foot self-care as an essential component of diabetes self-management at all levels of the health service delivery system.