1. Background
Klebsiella pneumoniae is an important opportunistic pathogen that causes various infections, such as urinary tract infections (UTIs), septicemia, and respiratory tract infections (1). Today, UTIs caused by multidrug-resistant (MDR) strains of K. pneumoniae are a major public health problem and a serious concern for the medical community.
Extended-spectrum beta-lactamase (ESBL)-producing isolates of K. pneumoniae are also considerate as major problems in hospitalized and community-based patients (2). ESBLs can hydrolyze a variety of β-lactam antibiotics, including cephalosporins (ceftazidime, cefotaxime, and ceftriaxone), monobactams (aztreonam), and penicillins, most of which are the widely used antibiotics in many hospitals. However, this class of beta-lactamases does not hydrolyze cephamycins such as cefoxitin (3).
Gram-negative beta-lactamases are categorized into four molecular groups, A - D. The group A enzymes include blaTEM and blaSHV families. TEM-1 and TEM-2 are two defined subtypes of TEM beta-lactamases (4). An important non-TEM, non-SHV group of ESBLs is CTX-M-1 that has hydrolytic activity against cefotaxime.
Another concern about resistance mechanisms in Gram-negative bacteria is the emergence of fluoroquinolone-resistant strains. Quinolone resistance is usually mediated by mutations in genes encoding DNA topoisomerase IV, DNA gyrase, and outer membrane proteins. Recently, however, plasmids carrying quinolone resistance determinants have been noticed as emerging clinical problems (5, 6) as they can be transmitted horizontally through bacterial conjugation.
Plasmid-mediated quinolone resistance (PMQR) can result from the pentapeptide-repeat proteins encoded by the qnr genes including qnrA, qnrB, qnrC, qnrD, and qnrS that protect the topoisomerase IV and DNA gyrase from quinolone inhibition (7). Another PMQR mechanism is an aminoglycoside acetyltransferase encoded by the aac(6’)-Ib-cr gene, which N-acetylates a piperazinyl amine substituent of some fluoroquinolones and decreases susceptibility to quinolones (7).
Recent studies documented the high prevalence of PMQR determinants among ESBL-producing K. pneumoniae isolates (8, 9). There is a little report of the prevalence of PMQR determinants in urinary isolates of ESBL-producing K. pneumoniae in Iran. Additionally, there are a few reports of the molecular typing of ESBL-producing K. pneumoniae isolates harboring PMQR genes.
2. Objectives
The goal of this study was to determine the frequency of PMQR determinants in ESBL-producing K. pneumoniae isolated from urine samples of patients admitted to Milad Hospital in Tehran, Iran. Molecular typing of ESBL-producing Klebsiella pneumoniae isolates was also investigated using enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR).
3. Methods
3.1. Bacterial Isolation
In this cross-sectional study, 100 strains of K. pneumoniae were obtained from patients with UTIs in Milad Hospital of Tehran between 2016 and 2018. All isolates were identified as K. pneumoniae using Gram-staining, culture characteristics, and biochemical tests (10).
3.2. Antibiotic Susceptibility Testing
Antibiotic susceptibility of K. pneumoniae isolates was determined by the Kirby Bauer disk diffusion method on Muller-Hinton agar (Merk, Germany) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (11). The applied antibiotic disks (MAST, UK) were meropenem (10 μg), imipenem (10 μg), gentamicin (30 μg), ceftazidime (30 μg), cefepime (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), amikacin (10 μg), ticarcillin (75 μg), and piperacillin (30 μg).
The phenotypic detection of ESBL-producing isolates was carried out using the combination disk method. The discs of ceftazidime and cefotaxime alone and combined with clavulanic acid (10 μg) were placed on the inoculated surface of Mueller-Hinton agar (Merk, Germany) plates by the standard disk diffusion method. The plates were then incubated overnight at 37ºC. An increase of ≥ 5 mm in the zone diameter of cefotaxime or ceftazidime combined with clavulanic acid versus cefotaxime or ceftazidime alone was considered as positive for ESBL (11).
3.3. Molecular Characterization of ESBL and Plasmid-Mediated Quinolone Resistance Genes
The DNA extraction was carried out using fresh overnight cultures of the strains in TSB by the SinaPure DNA extraction kit (Sinaclone, Iran). Extracted DNA samples from ESBL-producing isolates were examined by the multiplex PCR assay targeting three genes, blaSHV, blaTEM, blaCTX-M, and the simplex PCR assay for aac(6’)-Ib-cr, qnrA, qnrB, qnrC, qnrD, and qnrS genes (Table 1). The PCR mixture (25 μL) contained 3 - 5 μL of template DNA, 2.5 μL of 10X PCR buffer, 0.5 μL of 10 mM dNTPs, 0.75 μL of 50 mM MgCl2, 0.25 μL of 5 U/μL Taq DNA polymerase (Ampliqon, Denmark), and 10 pmol of each primer. The PCR conditions were as follows: initial denaturation at 94ºC for 5 min, followed by denaturation at 94ºC for 1 min, annealing at 61ºC for 1 min for beta-lactamase-related genes and 57ºC for 1.5 min for PMQR genes, extension at 72ºC for 2 min (30 cycles), and a final extension at 72ºC for 7 min.
| Target | Sequence (5’ - 3’) | Size (bp) | Reference |
|---|---|---|---|
| blaSHV | 474 | (12) | |
| ATGCGTTATATTCGCCTGTG | |||
| TGCTTTGTTATTCGGGCCAA | |||
| blaTEM | 445 | (12) | |
| TCGCCGCATACACTATTCTCAGAATGA | |||
| ACGCTCACCGGCTCCAGATTTAT | |||
| blaCTX-M | 593 | (12) | |
| ATGTGCAGCACCAGTAAAGTGATGGC | |||
| TGGGTAAAGTAAGTGACCAGAATCAGCGG | |||
| qnrA | 630 | (13) | |
| CAGCAAGAGGATTTCTCACG | |||
| AATCCGGCAGCACTATTACTC | |||
| qnrB | 448 | (13) | |
| GGCTGTCAGTTCTATGATCG | |||
| GAGCAACGATGCCTGGTAG | |||
| qnrC | 118 | (13) | |
| GCAGAATTCAGGGGTGTGAT | |||
| AACTGCTCCAAAAGCTGCTC | |||
| qnrD | 581 | (13) | |
| CGAGATCAATTTACGGGGAATA | |||
| AACAAGCTGAAGCGCCTG | |||
| qnrS | 428 | (13) | |
| GCAAGTTCATTGAACAGGGT | |||
| TCTAAACCGTCGAGTTCGGCG | |||
| aac(6')-Ib-cr | 208 | (13) | |
| TTGGAAGCGGGGACGGAM | |||
| ACACGGCTGGACCATA |
Sequences of Used Primers in This Study
3.4. Molecular Typing of ESBL-Producing Klebsiella pneumoniae Isolates
ERIC-PCR was used to determine the clonal relationships among the ESBL-producing K. pneumoniae isolates using the ERIC2 primer (5’-AAGTAAGTGACTGGGGTGAGCG-3’) as previously described (14). The dendrogram was constructed by an unweighted pair group method using arithmetic averages (UPGMA). The similarity cutoff level was set at 85%.
3.5. Statistical Analysis
Data were analyzed using SPSS version 20. Differences in categorical variables and susceptibility pattern between ESBL and non-ESBL-producing isolates were statistically analyzed by the chi-square test. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. P values of < 0.05 were considered significant.
4. Results
Out of 100 K. pneumoniae strains included in our study, 50 were obtained from females and 50 from males with an overall mean age of 46.96 ± 22.98 years. The highest susceptibility rates were observed to ticarcillin (96%) and imipenem (83%) (Table 2). These antibiotics were found as the most effective drugs against K. pneumoniae clinical isolates. The total results of antibiotic susceptibility testing for K. pneumoniae isolates are presented in Table 2. Further analysis revealed that the rate of ESBL-producing isolates was 51% (51/100).
| Antimicrobial Category | Antimicrobial Agents | K. pneumoniae, No. (%) | P Value | ||
|---|---|---|---|---|---|
| Total (N = 100) | Non-ESBL-Producing (N = 49) | ESBL-Producing (N = 51) | |||
| Aminoglycosides | |||||
| Gentamicin | 44 (44) | 27 (55.1) | 17 (33) | 0.023 | |
| Amikacin | 29 (29) | 15 (30.6) | 13 (25.5) | 0.36 | |
| Carbapenems | |||||
| Imipenem | 83 (83) | 45 (91.8) | 38 (74.5) | 0.019 | |
| Meropenem | 59 (59) | 30 (61.2) | 29 (56.9) | 0.405 | |
| Cephalosporins | |||||
| Cefepime | 36 (36) | 22 (44.9) | 12 (23.5) | 0.02 | |
| Ceftazidime | 41 (41) | 15 (30.6) | 26 (50.9) | 0.014 | |
| Fluoroquinolones | |||||
| Ciprofloxacin | 36 (36) | 24 (48.9) | 15 (29.4) | 0.036 | |
| Levofloxacin | 31 (31) | 16 (32.65) | 15 (29.4) | 0.44 | |
| Ureidopenicillins | Piperacillin | 41 (41) | 22 (44.9) | 19 | 0.283 |
| Carboxypenicillins | Ticarcillin | 96 (96) | 48 (97.9) | 48 (94.1) | 0.324 |
| Monobactams | Aztreonam | 97 (97) | 49 | 48 (94.1) | 0.129 |
Antibiotic Susceptibility Pattern of K. pneumoniae Isolates
We observed a significant difference between ESBL producers and non-ESBL producers in antibiotic susceptibility to gentamicin, imipenem, ceftazidime, cefepime, and ciprofloxacin (Table 2). Of the ESBL producers, 31 (60.8%) were multidrug-resistant (MDR), whereas only 20 (40.8%) of the non-ESBL producers were MDR strains. The difference was statistically significant (P < 0.05).
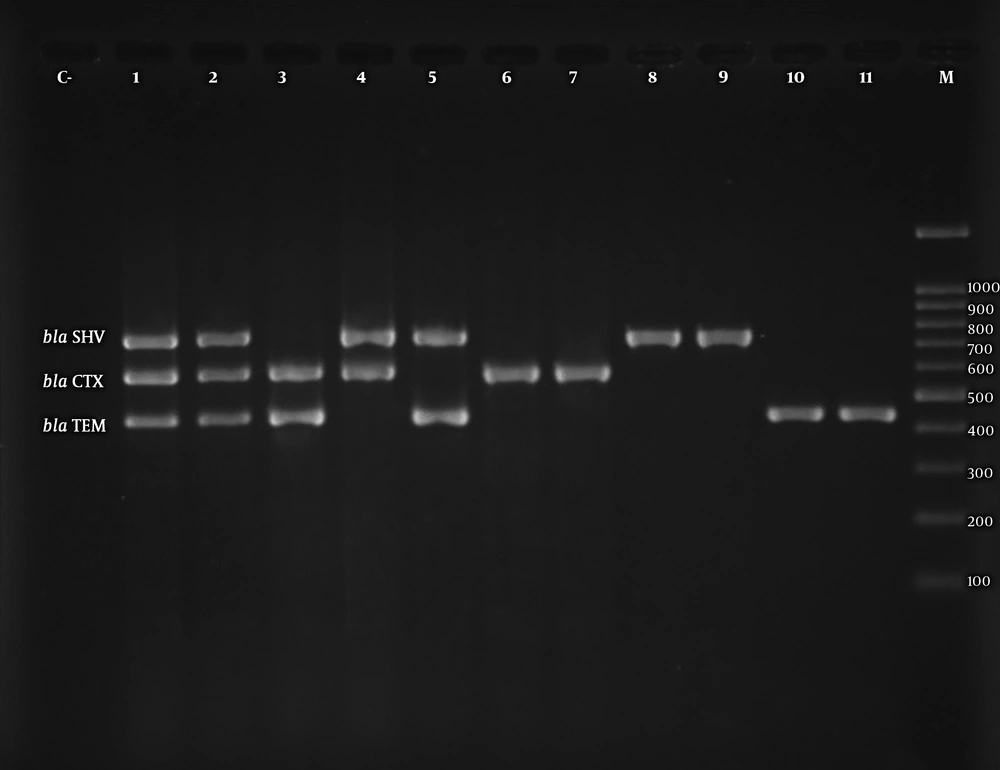
The ESBL-producing K. pneumoniae isolates were assessed by PCR for the detection of blaCTX-M, blaTEM, blaSHV, and plasmid-mediated quinolone resistance determinants. According to the PCR results, among 51 ESBL-positive isolates, blaTEM was the most prevalent gene (n = 48, 94.1%), followed by blaSHV (n = 34, 66.7%) and blaCTXM (n = 29, 59.9%). Among the ESBL-producing isolates, 27 (52.9%) and 16 (31.4%) isolates carried two and three types of ESBL genes, respectively.
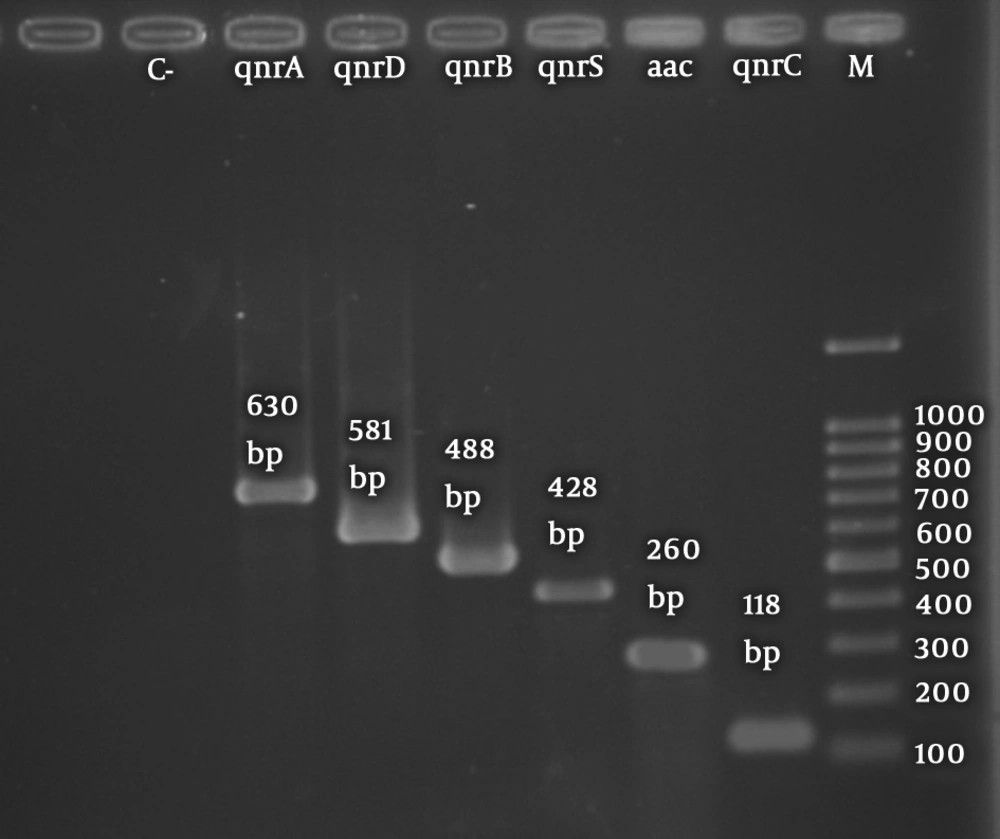
The aac(6’)-Ib gene was the most frequent PMQR gene in the urinary isolates of K. pneumoniae. Among 51 ESBL-producing isolates, aac(6’)-Ib was detected in 18 isolates (35.3%), qnrS in 17 isolates (33.3%), qnrD in 16 isolates (31.4%), qnrB in 15 isolates (29.4%), qnrA in 7 isolates (13.7%), and qnrC in 3 isolates (5.9%). Among 44 PMQR-positive isolates, 9 (21.9%) and 16 (39%) harbored 2 and 3 different PMQR determinants, respectively. The coexistence of ESBL and PMQR genes was observed in 44 (86.2%) isolates. Figures 1 and 2 display the electrophoretic patterns of ESBL and PMQR genes.
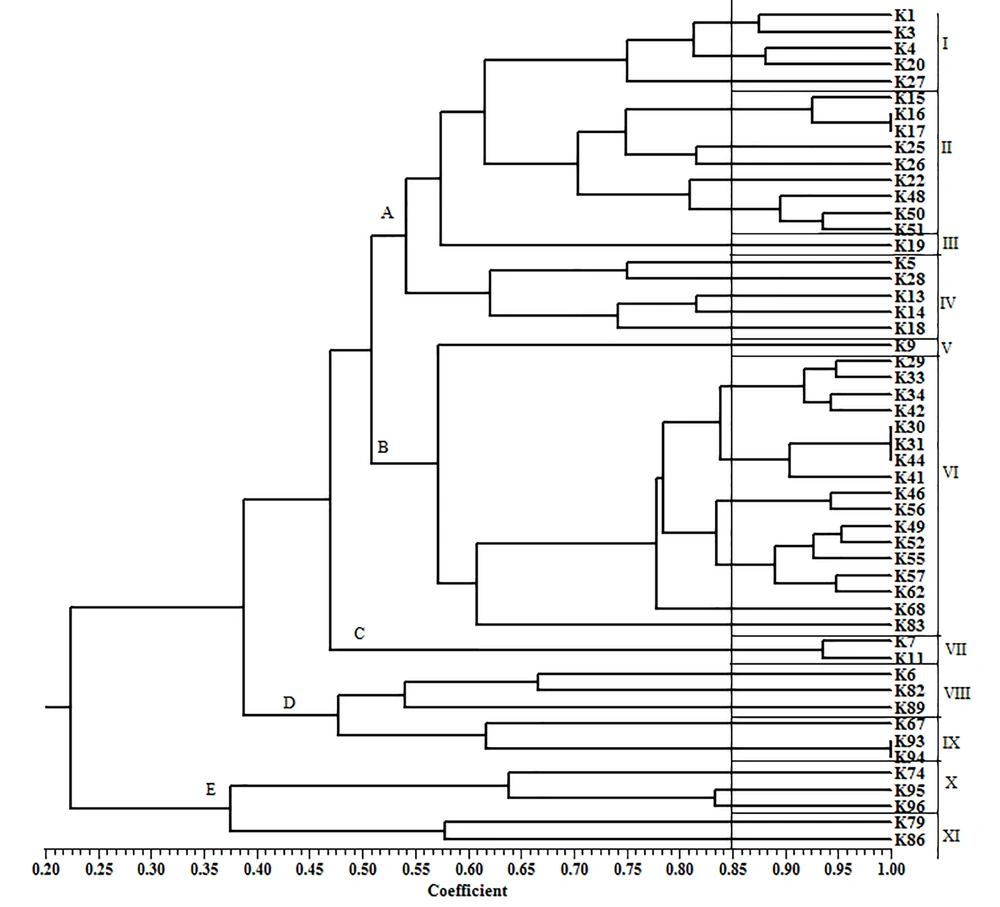
The ERIC-PCR dendrogram of ESBL-producing K. pneumoniae isolates is illustrated in Figure 3. The dendrogram revealed 5 major clusters and 11 different clonal types on a similarity level of 85% (Figure 3). From 51 ESBL-producing K. pneumoniae isolates, 20 strains were found in cluster A with 4 clonal types. This cluster contained the majority of ESBL-producing isolates (39.2%). ERIC-PCR indicated a heterogeneity among K. pneumoniae isolates.
5. Discussion
ESBL-producing K. pneumoniae isolates are of great concern, both in treatment and in management of nosocomial infections. As multiple resistance genes mostly reside on the same plasmid, these strains are usually multidrug-resistant (15). Plasmid-mediated resistance genes can be horizontally transmitted to other isolates and cause a high rate of mortality among patients affected by such strains. Continuous surveillance for ESBL-producing K. pneumoniae and resistance monitoring are absolutely necessary for infection control policies and treatment options.
The frequency of ESBL-producing K. pneumoniae isolates was higher in this study than in some previous Iranian studies (16-18). However, higher frequencies were reported by Mahmoudi et al. (97%) and Shakib et al. (69. 23%) (19, 20). Reports from some Asian countries demonstrate a comparable prevalence of ESBL-producing K. pneumoniae (21, 22). A higher prevalence was reported in India (68%); however, the frequencies mentioned in Korea (26.5%), Saudi Arabia (30%), and China (33.3%) were lower (23-26).
In our study, the frequency of ESBLs was significantly higher in isolates from adult patients of > 60-years-old (23 of 36, 63.9%; RR = 2.275. 95% CI = 0.98-5.27, P = 0.042) (Table 3). Our results are in agreement with a study conducted by Obeng-Nkrumah et al. (27). This may be due to the higher antibiotic pressure in elderly patients.
| Variable | ESBL, No. (%) (N = 51) | Non-ESBL, No. (%) (N = 49) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female (n = 50) | 30 (60) | 20 (40) | 1.905 (0.861 - 4.216) | |
| Male (n = 50) | 21 (42) | 29 (58) | 1.905 (0.861 - 4.216) | 0.08 |
| Age | ||||
| 1 - 10 (n = 13) | 6 (46) | 7 (53) | 0.8 (0.249 - 2.574) | 0.469 |
| 11 - 30 (n = 14) | 4 (28.6) | 10 (71.4) | 0.332 (0.097 - 1.141) | 0.063 |
| 31 - 60 (n = 37) | 18 (48.6) | 19 (51.4) | 0.861 (0.382 - 1.941) | 0.439 |
| > 60 (n = 36) | 23 (63.9) | 13 (36.1) | 2.275 (0.982 - 5.272) | 0.042 |
Demographic Factors Associated with ESBL Infections in Milad Hospital, Tehran, Iran
In the present study, we found that resistance to some of the tested antimicrobial agents was significantly higher in ESBL producers than in non-ESBL producers. As expected, ESBL-producing isolates showed a significantly higher resistance rate to cephalosporins and carbapenems. One of the most alarming properties of ESBL-positive K. pneumoniae isolates is the high rates of resistance to non-beta lactam antimicrobials, especially aminoglycosides and quinolones. In a study from Iran in 2018, ciprofloxacin resistance was reported to be 59.3% in ESBL producers and 39.4% in non-ESBL K. pneumoniae isolates (17). Higher rates of ciprofloxacin resistance in ESBL-producing K. pneumoniae isolates were reported in Korea and India (80.9% and 85%, respectively) (24, 28). Our findings represent a high frequency of ciprofloxacin resistance (70.6%) among ESBL-positive K. pneumoniae isolates.
A remarkable point in the present study is the relatively high frequency of imipenem resistance in ESBL producers, which was rarely reported in previous research in Iran. In a meta-analysis study conducted by Heidary et al. in Iran, the imipenem resistance in K. pneumoniae isolates was reported as 3.2% (29). Our data represent a sharp rise in imipenem resistance among K. pneumoniae isolates. The spread of such strains in hospital settings could further limit treatment options for UTIs.
The genotype of ESBL-producing isolates varies between countries and even hospital settings in which they are isolated. Our findings indicated that blaTEM was more prevalent (94%) than blaSHV (66.7%) and blaCTXM (59.9%). Contrary to our results, Ghafourian et al. and Eftekhar et al. reported blaSHV as the predominant bla gene among ESBL-producing isolates (30, 31). In Syria, blaCTX-M-1 was the commonest genotype (100%), followed by blaSHV (92.59%) and blaTEM (59,59%) (32). In India, blaTEM was the predominant gene (52%), followed by blaSHV (45%) and blaCTX-M (37 %) (28). In Saudi Arabia, blaSHV gene (89.1%) predominated, followed by blaTEM (70.9%) (33) and in Egypt, blaCTXM-positive K. pneumoniae isolates were more prevalent than blaSHV-positive isolates (34). It can be concluded that prescribed antibiotics in each hospital and region can affect the distribution of ESBL genotypes.
Our results indicated a high prevalence (86.3%) of PMQR determinants among 51 ESBL-positive strains of K. pneumoniae that was comparable with a previous study conducted by Shams et al. in Kashan, Iran (35). Similar to our results, they reported acc(6’)-Ib-cr as the predominant PMQR gene. The frequency of qnr genes was much higher in the present study (80.4%) than in studies from Korea (40.5%), Morocco (50.0%), Tunisia (15.0%), Mexico (13.7%), and Iran (8, 24, 35-38). In this study, the most frequent qnr gene among ESBL isolates was qnrS, followed by qnrD and qnrB. However, in previous studies conducted in Iran, qnrB was found to be as the most prevalent qnr gene (35). Our findings represented high rates of TEM, SHV, and CTXM beta-lactamases in qnr-positive isolates. The association of qnr genes with ESBL genes has been described in several studies (39).
The results of ERIC-PCR showed high rates of heterogeneity among ESBL-producing isolates. Therefore, the ESBL genes probably had been spread through the transfer of resistance determinants among the isolates. Previously, Eftekhar and Nouri also showed high rates of heterogeneity among ESBL-producing K. pneumoniae isolates (40). However, clonal dissemination of ESBL-producing K. pneumoniae isolates had been reported by Ghaffarian et al. in the north of Iran (17).
5.1. Conclusions
This study showed a high prevalence of ESBL and PMQR genes among ESBL-producing K. pneumoniae isolates in the studied hospital. Antibiotic resistance and MDR were significantly higher in ESBL producers than in non-ESBL producers. ERIC-PCR analysis showed the probable horizontal transfer of resistance determinants among the isolates. Our findings raise concerns about the dissemination of ESBL-producing K. pneumoniae isolates and highlight the need for ESBL detection and continuous monitoring of their resistance patterns in routine laboratories.