1. Background
The lipids are heterogeneous and hydrophobic compounds, having different biological functions such as structural components of cell membranes, energy, and signaling pathways. The lipid metabolites and pathway strategy (MAPS) classification system is comprised of eight lipid categories, fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, polyketides, sterol, and prenol lipids (1). Lipoproteins are large macromolecular compounds that transport poorly soluble lipids, including fat-soluble vitamins, cholesterol, and triglycerides (TG) that according to density and size, are divided into chylomicrons, very low-density lipoprotein (VLDL-C), intermediate-density lipoproteins (IDL-C), low-density lipoproteins (LDL-C), and high-density lipoprotein (HDL-C) (2).
Triacylglycerol is the dominant fat of diet about 90% - 95% of the total energy derived from dietary fat. There were various steps in lipid digestion, absorption, and metabolism, and this is important in developing drugs to reduce the risk of lipid-associated disorders (3). Dyslipidemia is one of the most important risk factors for cardiovascular diseases (CVD). The HDL-C has a strong inverse correlation with CVD risk, while LDL-C is the most atherogenic lipoprotein (4). In the last decades, triglycerides were found to be capable of entering the arterial wall like other dangerous lipids (5) and associated with proinflammatory effects, impaired fibrinolysis (6, 7), endothelial dysfunction (8), an increase in the LDL particles (9).
Lipid profile is usually measured in fasting state, the first reason is that increasing in TG level and decreasing in LDL-C level in non-fasting state. The maximum increase in TG level is measured by fat tolerance test (10) (mean fasting for 8 hours and then consumption about 70 - 80 gram of fat, then measuring TG after 4 hours) (5) and this amount of fat is usually ingested during a whole day of most individual, so changes in TG level will be minimally increased after habitual food intake (11-13). The common practice of measuring lipids in the fasting state may have some limitations. First, using non-fasting lipid profiles will provide more compliance for the patients, may prevent the delay in the decision of starting treatment, and reduce the workloads in the laboratory. Second, most people usually present in the non-fasting state (3 meals and snacks). So fasting lipid profiles will not reflect daily lipid in the body (14). Finally, in two large studies (15, 16), women’s health study and Copenhagen City Heart study, both showed that non-fasting TG independently was associated with CVD. This finding supports the importance of measuring lipids, especially TG, in the non-fasting state.
2. Objectives
The aim of this study was to evaluate whether there is any change in the measurement of lipid profile in fasting and non-fasting states and its effect on patients’ management.
3. Methods
A cross-sectional observational study was performed from January to November 2017 involving out-patients attending Faiha Specialized Diabetes, Endocrine and Metabolism Center (FDEMC) and Basrah General Hospital for a routine checkup. The Ethics Committee of FDEMC approved the study under reference number 23-22/01/2017. One hundred ninety-four patients aged from 20 to 78 years enrolled in the study, 102 (52.6%) were female and 92 (47.4%) were male.
Clinical data were taken from the patients in the form of age, gender, and body mass index (BMI), which was calculated as the body weight in kilogram divided by the squared body height in meter. The patients were divided into normal BMI < 25 kg/m2, overweight 25 - 29.9, obese ≥ 30 kg/m2 (17). Smoking history was classified as current smokers who had smoked greater than 100 cigarettes in their lifetime and continue till now, and non-smokers including both ex-smokers and never smokers (according to centers for disease control and prevention). The patients were considered to have hypertension (HTN) if they were known hypertensive on antihypertensive treatments or had systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg measured on two occasions (18). The patients were considered to have diabetes mellitus (DM) if they were known to have diabetes or met the American Diabetes Association diagnostic criteria 2017 (19). History of chronic kidney diseases (CKD) (self-reported or by estimated glomerular filtration rate (eGFR), CKD was defined by the presence of abnormalities of kidney function or structure that persist for 3 months) (20). Also, the history of statin use (more than one month's duration), and the history of having cardiovascular diseases (CVD) in the form of coronary vascular, cerebrovascular, and peripheral vascular diseases.
We have classified our patients according to the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) into atherosclerotic CVD risk categories in the form of an extreme, very high, high, moderate and low risk (21).
3.1. Exclusion Criteria
Patients’ age < 18, pregnancy, on medications that cause severe hypertriglyceridemia, including corticosteroids, estrogens, tamoxifen, isotretinoin, bile acid-binding resins, phenothiazines, and other second-generation antipsychotics.
Blood samples were drawn from the patients for the measurement of lipid profile (TC, TG, VLDL-C, LDL-C, HDL-C, and non-HDL cholesterol). Here, LDL-C was directly measured, while non-HDL-C was calculated as (total cholesterol minus HDL). About five ml of blood was drawn in gel and clot activator then centrifuged and put in plain tube. The analysis was done by COBAS (INTEGRA 400 PLUS). Two samples of blood were drawn from each patient, the first was a non-fasting sample, and the second was a fasting sample (at least 8 hours no eating or drinking even water) and 24 hours apart from the first sample. Glycated hemoglobin (HbA1C) was drawn for the patients into ethylenediaminetetraacetic acid (EDTA)-containing tubes and measured by high-performance liquid chromatography (HPLC) using BIO-RAD D-10. Blood urea and serum creatinine were measured with the same machine used for lipid profile.
The Statistical Package for the Social Sciences (SPSS) version 23 was used for analysis. Continuous variables were summarized as mean and standard deviation. Categorical variables were summarized as numbers and frequencies. Independent student t-test was used for comparison of lipid data between fasting and non-fasting states. The correlation between categorical variables was done using Chi-square test. A P value of < 0.05 was considered statistically significant.
4. Results
The mean age was 50.4 ± 11.7 years. One hundred and two (52.6%) patients were diabetic, mean duration 8.15 ± 6.3 years, mean HbA1c 9.5 ± 2.23 percentages. Eighty-six (44.3%) patients were hypertensive, 50 (25.8%) patients had CVD, 24 (12.4%) patients were a current smoker. Chronic kidney diseases were present in 4 (2.1%) and the mean BMI was 28.4 ± 5.7 (Table 1).
| Variable | |
|---|---|
| Age, y | 50.4 ± 11.7 |
| ≥ 55 | 78 (40.2) |
| < 55 | 116 (59.8) |
| BMI, kg/m2 | 28.4 ± 5.7 |
| Normal | 56 (28.9) |
| Overweight | 60 (30.9) |
| Obese | 78 (40.2) |
| Gender | |
| Male | 92 (47.4) |
| Female | 102 (52.6) |
| Smoking | 24 (12.4) |
| HTN | 86 (44.3) |
| CVD | 50 (25.8) |
| CKD | 4 (2.1) |
| DM | 102 (52.6) |
| Duration, y | 8.15 ± 6.3 |
| HbA1c, % | 9.5 ± 2.23 |
| < 7b | 10 (12.2) |
| > 7 | 72 (87.8) |
| Statin | 62 (32) |
| CVD risk category | |
| Low | 25 (12.9) |
| Moderate | 31 (16) |
| High | 28 (14.4) |
| Very high | 82 (42.3) |
| Extreme | 28 (14.4) |
Abbreviations: BMI, body mass index; CKD, chronic kidney diseases; CVD, cardiovascular disease; DM, diabetes mellitus; HTN, hypertension.
aValues are expressed as No. (%) or mean ± SD.
bFrequency for diabetic only and within those having their HbA1c measured within the last three months.
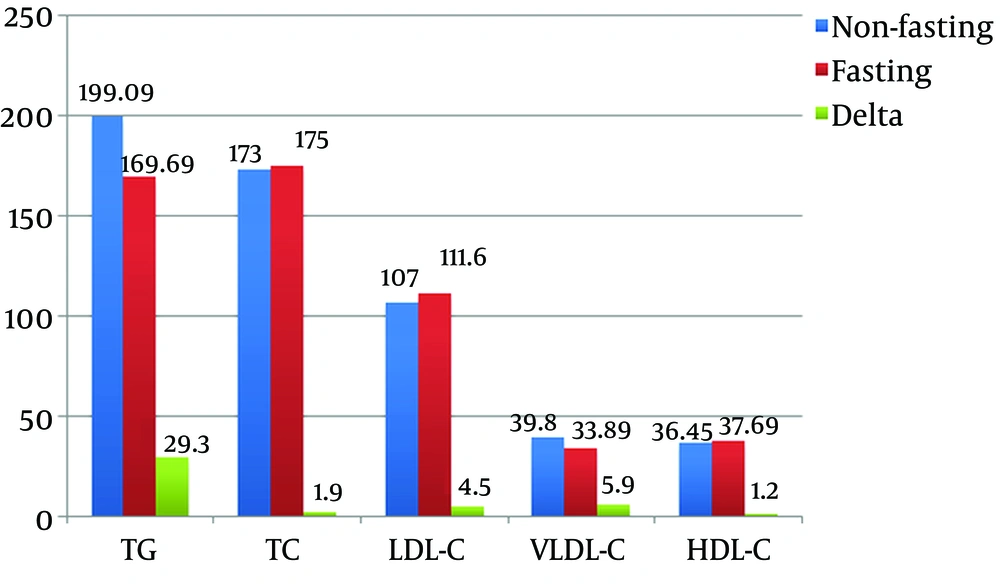
Comparison of the measurements of lipid profiles of the patients showed that TG and VLDL-C were significantly higher in the non-fasting compared to the fasting state (P = 0.004). The mean TG were 199.09 ± 111.13 mg/dL (2.25 ± 1.26 mmol/L) and 169.6 ± 88.5 mg/dL (1.92 ± 1 mmol/L) in non-fasting and fasting states, respectively. The mean VLDL-C were 39.8 ± 22.1 mg/dL (1.03 ± 0.57 mmol/L) and 33.8 ± 17.6 mg/dL (0.88 ± 0.46 mmol/L) in non-fasting and fasting states, respectively. The mean change TG was 29.39 ± 60 mg/dL (0.3 ± 0.6 mmol/L), and the mean change VLDL-C was 5.9 ± 12.3 mg/dL (0.15 ± 0.31 mmol/L). While for TC, LDL-C, and HDL-C showed no significant differences (Figure 1).
In Table 2, the patients were divided into groups according to age, gender, BMI, smoking status, DM, HTN, CVD, and statin use. While there were no significant different TC, LDL-C, and HDL-C between fasting and non-fasting states, a significantly higher TG and VLDL-C was found in non-fasting state in patients aged < 55 years (P = 0.004, 0.004), women (P = 0.019, 0.018), obese patients (P = 0.019, 0.018), non-smokers (P = 0.01, 0.01), diabetics (P = 0.007, 0.006), hypertensive (P = 0.02, 0.02), non-CVD patients (P = 0.002, 0.002), and patients without statin consumption (P = 0.029, 0.03). For older patients, men, non-obese, smokers, non-diabetics, non-hypertensive, patients with CVD, and statin users, both TG and VLDL-C showed no significant difference.
| Variable | TG | TC | LDL-C | VLDL-C | HDL-C |
|---|---|---|---|---|---|
| Age < 55 | |||||
| Fasting | 174.1 ± 92.6 | 181.7 ± 50.7 | 117.5 ± 44 | 34.7 ± 18.4 | 37.9 ± 14.8 |
| Non | 213.2 ± 112 | 181.3 ± 51 | 112.6 ± 46.6 | 42.6 ± 22.5 | 36.2 ± 14.1 |
| P value | 0.004 | 0.9 | 0.4 | 0.004 | 0.3 |
| Age ≥ 55 | |||||
| Fasting | 163 ± 82.1 | 167.1 ± 52.3 | 102.9 ± 48.8 | 32.5 ± 16.3 | 37.2 ± 16.1 |
| Non | 178 ± 105.5 | 162.9 ± 51.6 | 98.7 ± 43.8 | 35.5 ± 21 | 36.7 ± 15.4 |
| P value | 0.3 | 0.6 | 0.5 | 0.3 | 0.8 |
| Men | |||||
| Fasting | 170.9 ± 84.5 | 168.2 ± 56 | 105 ± 50.5 | 34.1 ± 16.8 | 34.1 ± 15.5 |
| Non | 195.3 ± 111 | 165.7 ± 59.7 | 101.1 ± 52.6 | 39 ± 22.2 | 32.8 ± 15.2 |
| P value | 0.09 | 0.7 | 0.6 | 0.095 | 0.5 |
| Women | |||||
| Fasting | 168.5 ± 92.3 | 182.7 ± 46.7 | 117.6 ± 41.7 | 33.6 ± 18.4 | 40.8 ± 14.6 |
| Non | 202.4 ± 111 | 181.2 ± 42.7 | 112.4 ± 38.4 | 40.4 ± 22.2 | 39.6 ± 13.4 |
| P value | 0.019 | 0.8 | 0.3 | 0.018 | 0.5 |
| Normal weight | |||||
| Fasting | 160.8 ± 98.3 | 176.9 ± 63.5 | 111.4 ± 51.8 | 32 ± 19.6 | 37 ± 12.9 |
| Non | 183 ± 110.5 | 177.5 ± 69.7 | 111.9 ± 55.8 | 36.5 ± 22.1 | 36.8 ± 12.3 |
| P value | 0.2 | 0.9 | 0.9 | 0.2 | 0.9 |
| Overweight | |||||
| Fasting | 171 ± 79.7 | 172.9 ± 50.4 | 110.4 ± 46 | 34.1 ± 15.8 | 35.9 ± 16.7 |
| Non | 194.2 ± 103 | 169 ± 48.4 | 104.7 ± 47.3 | 38.7 ± 20.5 | 34.4 ± 16 |
| P value | 0.17 | 0.6 | 0.5 | 0.1 | 0.6 |
| Obese | |||||
| Fasting | 175 ± 88.1 | 177.3 ± 43.2 | 112.7 ± 43.1 | 34.9 ± 17.6 | 39.4 ± 15.8 |
| Non | 214.3 ± 116 | 175 ± 38.2 | 105.4 ± 36.4 | 42.8 ± 23.3 | 37.6 ± 15.1 |
| P value | 0.019 | 0.7 | 0.25 | 0.018 | 0.4 |
| Smoker | |||||
| Fasting | 181.2 ± 98 | 178.9 ± 52.6 | 115.9 ± 43.7 | 36.1 ± 19.5 | 33.6 ± 11.8 |
| Non | 223.3 ± 118 | 179.1 ± 48.2 | 111.7 ± 44.4 | 44.6 ± 23.6 | 30.5 ± 10.4 |
| P value | 0.18 | 0.9 | 0.7 | 0.18 | 0.34 |
| Non-smoker | |||||
| Fasting | 168 ± 87.2 | 175.4 ± 51.7 | 111 ± 46.9 | 33.5 ± 17.4 | 38.2 ± 15.7 |
| Non | 195.6 ± 109 | 173.1 ± 52.5 | 106.4 ± 46.2 | 39.1 ± 21.9 | 37.2 ± 15 |
| P value | 0.01 | 0.7 | 0.3 | 0.01 | 0.5 |
| Diabetic | |||||
| Fasting | 178.4 ± 90.2 | 177.7 ± 43.4 | 110.6 ± 38.9 | 35.6 ± 17.9 | 38.1 ± 13 |
| Non | 218.7 ± 118 | 175.1 ± 41.1 | 103.3 ± 35.6 | 43.7 ± 23.6 | 37.1 ± 12.1 |
| P value | 0.007 | 0.6 | 0.16 | 0.006 | 0.5 |
| Non-diabetic | |||||
| Fasting | 159.9 ± 85.9 | 173.7 ± 59.8 | 112.7 ± 53.7 | 31.9 ± 17.2 | 37.1 ± 17.6 |
| Non | 177.3 ± 98.5 | 172.5 ± 62 | 111.2 ± 55 | 35.4 ± 19.6 | 35.6 ± 17 |
| P value | 0.2 | 0.8 | 0.8 | 0.2 | 0.5 |
| HTN | |||||
| Fasting | 167 ± 72.5 | 172.7 ± 46.2 | 110.1 ± 43.9 | 33.3 ± 14.5 | 35.8 ± 13 |
| Non | 198 ± 104.3 | 170 ± 44.9 | 104.8 ± 40.6 | 39.6 ± 20.8 | 34.5 ± 12.6 |
| P value | 0.02 | 0.7 | 0.4 | 0.02 | 0.5 |
| No HTN | |||||
| Fasting | 171.8 ± 99.7 | 178.3 ± 55.8 | 112.8 ± 48.5 | 34.3 ± 19.8 | 39.1 ± 16.8 |
| Non | 199.8 ± 116 | 176.9 ± 56.9 | 108.8 ± 49.8 | 39.9 ± 23.2 | 37.9 ± 15.9 |
| P value | 0.059 | 0.8 | 0.5 | 0.056 | 0.6 |
| CVD | |||||
| Fasting | 177.4 ± 85.4 | 177.3 ± 40.8 | 113 ± 41.8 | 35.4 ± 17 | 37.6 ± 14.1 |
| Non | 192.3 ± 113 | 173.4 ± 43.9 | 112 ± 41.5 | 38.4 ± 22.7 | 36.5 ± 13.7 |
| P value | 0.46 | 0.6 | 0.9 | 0.45 | 0.7 |
| No CVD | |||||
| Fasting | 167.9 ± 86.5 | 176.2 ± 52.3 | 112.2 ± 46.6 | 33.5 ± 17.2 | 37.7 ± 15.2 |
| Non | 200.2 ± 111 | 174.2 ± 52.1 | 107.4 ± 46.1 | 40 ± 22.2 | 36.2 ± 14.6 |
| P value | 0.002 | 0.7 | 0.3 | 0.002 | 0.3 |
| Statins | |||||
| Fasting | 176.1 ± 97.9 | 173.8 ± 45.9 | 106.7 ± 42.3 | 35.1 ± 19.4 | 39.7 ± 15.3 |
| Non | 214.4 ± 130 | 172.5 ± 45.4 | 102.7 ± 39.6 | 42.8 ± 25.9 | 38.7 ± 14.2 |
| P value | 0.06 | 0.8 | 0.5 | 0.06 | 0.7 |
| No statin | |||||
| Fasting | 166.6 ± 83.9 | 176.8 ± 54.4 | 113.9 ± 48.2 | 33.2 ± 16.7 | 36.7 ± 15.3 |
| Non | 191.8 ± 100 | 174.5 ± 54.9 | 109.1 ± 48.6 | 38.3 ± 20.1 | 35.3 ± 14.7 |
| P value | 0.029 | 0.5 | 0.7 | 0.03 | 0.8 |
aValues are expressed as mean ± SD.
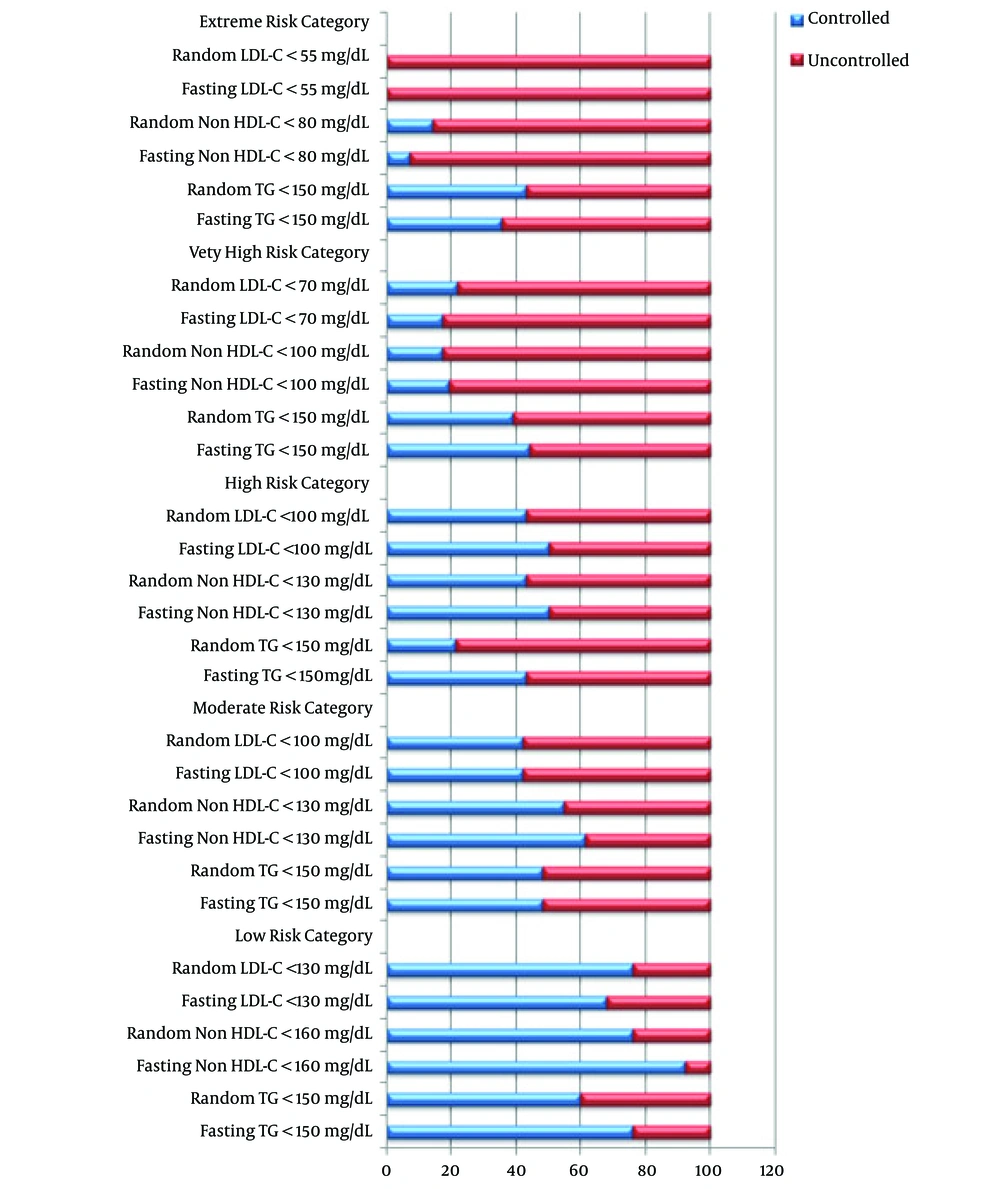
In Figure 2, which shows the percentage of achieved targeted lipid profiles according to different CVD risk categories, although the percentage of controlled lipids was higher in fasting state, especially for achieved target TG, measuring lipids in fasting and non-fasting state did not show a significant change in different CVD risk categories (P > 0.05).
5. Discussion
In this study, there were no significant changes between fasting and non-fasting level of each TC, LDL-C, and HDL-C, which were decreased in non-fasting state, while TG and VLDL-C were increased in a significant manner. In a study that was done in Denmark involving more than 33,000 men and women showed that the decrease in TC and LDL-C was due to hemodilution from fluid intake, while the HDL-C was decreased due to bidirectional lipid exchange, and TG was increased due to food intake mainly fat, both changes in HDL-C and TG are significant (11). A similar finding for HDL-C and TG was observed by Mora et al. (22).
The increase in TG in non-fasting state was only observed in patients aged < 55 years, while no significant changes were reported in older patients. Other studies found a link between aging and the magnitude of postprandial lipidemia and TG clearance, the main cause for these changes in non-fasting TG regarding age is not well established so far (22). The finding of a significant increase in TG and VLDL-C in non-fasting women only might be explained by fat body distribution. Abdominal fat has been inversely associated with the suppression of fatty acid release from adipocyte, and the free fatty acid is an important source of fatty acids for the assembly of VLDL-C. However, other studies found that non-fasting TG has stronger effects in predicting CVD among women than men (16, 23, 24).
The finding of increased non-fasting TG and VLDL-C level in diabetic patients was supported by two studies. Teno et al. (25) found that in diabetics, the non-fasting TG was significantly higher than fasting TG, and also found there was a correlation between increased concentrations of postprandial TG and increased carotid intima-media thickness among 61 patients with DM. Carstensen et al. (26) observed that non-fasting TG among 32 diabetic patients could be used as a predictor of myocardial infarction. No significant changes were found in the lipid profile in fasting and non-fasting regarding cofounders as smoking, which was in line with one study from North Europe (27), while there was a statistically significant increase in non-fasting TG in non-smokers, who were the vast majority of our patients.
No difference was found for those having CVD between fasting and non-fasting lipid profiles, which could be explained by that they were taking statins. The explanation for finding no difference in lipid profile in those taking statins may be the fact that statin improved postprandial lipoprotein metabolism (28). It was also found in this study that there was an increase in TG and VLDL-C in non-fasting state in patients with an increased BMI and HTN. This finding is similar to what was seen in other studies, which showed obesity, regardless of concomitant diseases (DM, familial hyperlipidemia and hypertension), aggravates TG (29, 30).
Two of the limitations of this study were that we did not study the impact of different types of food on non-fasting lipid profile, and the unclear interval between non-fasting blood sampling and food consumption. However, in conclusion, this study revealed a significant increase in the level of TG and VLDL-C in non-fasting state. In spite of the decrease in LDL-C, HDL-C, and TC in the non-fasting state, these changes were minimal and not significantly affected by fasting. Measuring lipid profile in the non-fasting state did not affect the cardiovascular risk group classification while reducing the burden on both patients and health care services.