1. Context
Procedural sedation and analgesia (PSA) is commonly used in emergency departments (EDs) to facilitate the implementation of potentially painful procedures (1). Patients undergoing painful procedures need moderate or deep procedural sedation to achieve appropriate therapeutic outcomes; accordingly, the provision of all levels of sedation in ED is necessary (2). The PSA can be induced by a wide range of medications, such as midazolam, etomidate, propofol, fentanyl, ketamine, and propofol/ketamine-i e, ketofol. Some short-acting sedative agents, such as propofol (3), etomidate, (4), and ketamine, are extensively applied for PSA in EDs (3). These agents allow patients to maintain their airway reflexes and respond to verbal stimuli. In this regard, the use of short-acting sedative agents that provide adequate sedation with minimum adverse effects is optimal (5).
Propofol is a non-opioid, non-barbiturate, sedative-hypnotic agent that has a rapid onset of action and short recovery time (6). The onset time of propofol action is approximately 45 seconds, and its redistribution time from the blood to the fat and muscles ranges 3 - 5 minutes (5, 7, 8). Ketamine is an analgesic and sedative agent with amnestic properties, derived from phencyclidine. This agent facilitates the preservation of the muscle tone and protection of the airway reflexes and spontaneous respiration (9). Moreover, ketamine is effective in preventing injection pain (10, 11) and improving hemodynamic depression caused by propofol (12). The possible side effects of ketamine include emergence phenomena, postoperative dysphoria, vomiting, or laryngospasm (13-15). However, the combined use of ketamine and propofol can decrease the dose-dependent side effects of these agents (15). Ketofol is commonly used in the bolus form in EDs, operating rooms, and ambulatory settings (15-17).
Etomidate is another ultrashort-acting sedative agent with an action onset of approximately one minute and an action duration of 5 - 15 minutes. This sedative has the least hemodynamic effect in comparison with other PSA agents (5). The main problems of this agent include painful infusion and transient cognitive dysfunction. Benzodiazepines, such as midazolam, are common agents applied for the induction of PSA. Respiratory depression is the core adverse reaction of midazolam (18). Fentanyl is an ultrashort-acting sedative, which is used in combination with another agent for PSA. In this regard, a combination of fentanyl and propofol is used to prevent dyspareunia since propofol exerts no analgesic effect (19).
There are several guidelines for the use of analgesic, sedative, and anesthetic agents (1, 20, 21) in different combinations for PSA (22, 23). The pillar clinical policy on PSA is developed by the American College of Emergency Physicians (ACEP) (1). However, there are still reports on the incidence of various adverse events due to PSA in EDs. Given the importance of monitoring patients in ED and selecting an appropriate medication under such circumstances, the present study aimed at comparing the efficacy of sedatives applied for PSA in EDs. The study findings would be useful for emergency physicians to make a proper decision regarding the selection of sedatives for PSA.
2. Methods
The present systematic review was conducted to investigate the evidence of proper dosage, adverse events, sedation, and recovery time of the commonly used sedatives in EDs. To this end, a comprehensive search was conducted for articles addressing the effectiveness of midazolam, etomidate, propofol, fentanyl, ketamine, and propofol/ketamine (i e, ketofol) for the induction of PSA. The research stages, including question formation, eligibility criteria inspection, search implementation, article selection, determination of article assessment criteria, information extraction, and discussion, were designed based on the Cochrane Handbook (24). A specified protocol was also designed before the initiation of the study.
2.1. Eligibility Criteria
The current systematic review was conducted on original, randomized, controlled trials, in which PSA was induced in adults admitted to ED. Therefore, prospective observational, in-vitro, and retrospective studies were excluded from the study. The inclusion criteria were: (1) a clear description of the treatment protocol, (2) provision of objective results and measurements, and (3) publication in the English language. The exclusion criteria entailed: (1) investigation of subjects under 14 years, (2) insufficient data, (3) inclusion of animal samples, (4) examination of medications other than midazolam, etomidate, propofol, fentanyl, ketamine, and ketofol for PSA, and (5) sample size smaller than 40. Moreover, meta-analyses, expert opinions, letters to the editor, case reports/case series, consensus statements, and qualitative studies were excluded from the review. Moderate and deep sedation was defined based on the Clinical Policy of ACEP in all studies.
2.2. Types of Medications, Procedures, and Interventions
The medications used for the induction of moderate to deep PSA were: midazolam, etomidate, propofol, fentanyl, ketamine, and ketofol. Some of the procedures for which PAS was applied were: shoulder reduction, orthopedic joint or fracture reductions, repair of deep traumatic lacerations, the reduction of bone fractures, chest tube insertion, electrical cardioversion, incision and drainage of abscesses, tibial traction pin placement, chest tube thoracostomy, foreign body removal, upper endoscopy, lumbar puncture, hemorrhoidectomy, suturing, burn wound care, hernia reduction, fecal disimpaction, urinary catheter placement, central line placement, nasopharyngoscopy, pelvic examination, cervical dilation, and curettage.
In all the studies, the sedation had been performed in EDs by emergency physicians or care providers, including nurse practitioners or physician assistants. Sedatives were applied alone or in combination either intravenously (IV) or intramuscularly (IM). The post-PSA measures and pre-PSA medications (e g, opioids) were not considered as interventions. The protocol of each research was used to monitor the patients undergoing PSA.
2.3. Literature Search, Study Selection, and Data Extraction
The relevant articles published up to 2019 were searched in four electronic databases, including Google Scholar, PubMed, Web of Science, Medline, Scopus, and Embase. The search process was accomplished using the keywords Midazolam, Etomidate, Propofol, Fentanyl, Ketamine, and Ketofol in combination with Procedural Sedation, Analgesia, and Emergency Department. The search process was performed by two researchers and a senior expert researcher. The researchers were continuously in contact with each other to exchange information and select the eligible papers. In the first stage, all the titles and abstracts were evaluated and screened based on the eligibility criteria by the two investigators. In the next stage, the full-text versions of the papers related to the research objective were retrieved and evaluated for eligibility by two independent investigators.
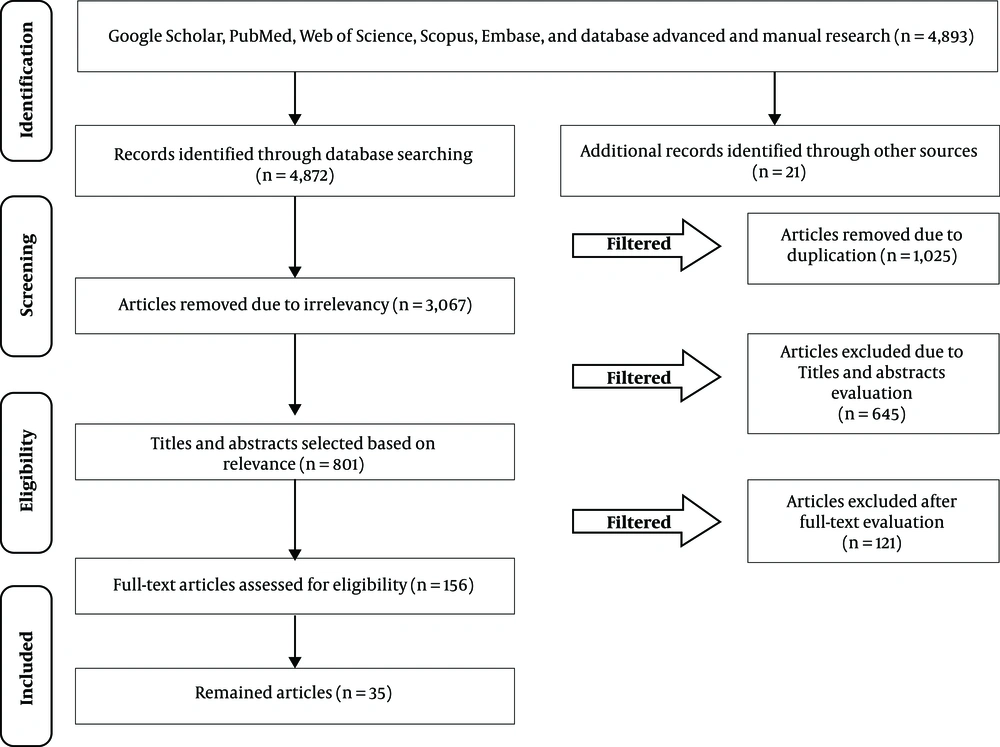
After screening the titles, papers comparing medicines other than midazolam, etomidate, propofol, fentanyl, ketamine, and ketofol, were excluded. Any disagreements were resolved through discussion. The remaining papers were subjected to a meticulous review. A standardized form was designed to record the data of each paper separately. Two reviewers assessed the extracted data and reached a consensus on the selected data. The last search was carried out on 01 May 2019. The recorded information included the applied medications, sample size, participants’ age, female to male ratio, total dosage, systolic blood pressure, oxygen desaturation, emergence reaction (agitation), respiratory adverse events (e g, use of a bag valve mask), sedation duration, time to recovery, and the pain scale. Figure 1 depicts the PRISMA flowchart of the article selection process.
2.4. Risk of Bias in Individual Trials
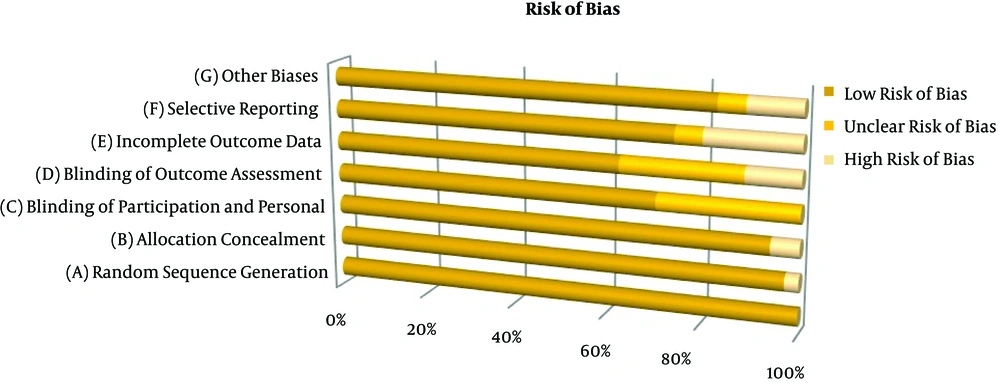
The Cochrane Collaboration tool was used to evaluate the risk of bias in the included trials (25, 26). The current review solely focused on the blinded, randomized, controlled, clinical trials. The clinical heterogeneity of the papers was evaluated in terms of the subjects’ characteristics, interventions, outcomes, and timing of outcome measurement. In order to improve the quality of the review, the data were extracted by two researchers who agreed on the eligibility criteria and resolved their disagreements through discussion. The articles with missing data were classified as unclear.
3. Results
3.1. Description of Included Studies
The search process resulted in the identification of 4893 articles, of which 3067 and 1025 were removed after initial evaluation due to being irrelevant and duplicate, respectively. Out of the remaining 801 papers, 766 did not meet the inclusion criteria; therefore, a total of 35 articles were selected for the final analysis (Figure 1).
3.2. Study Characteristics
The reasons for the removal of 766 articles included the enrollment of pediatric patients (n = 364), the examination of other sedative medications (n = 32), lack of investigating the drug effect (n = 19), lack of implementing in an ED (n = 8), a sample size smaller than 40 (n = 3), publication in non-English languages (n = 3), and inaccessibility of the full-text version (n = 3). In addition, experimental (n = 3), descriptive and cross sectional (n = 29), prospective observational and cohort (n = 58), and observational pilot studies (n = 11), as well as nonrandomized or non-blinded prospective trials (n = 15), were excluded from the study. Moreover, retrospective studies (n = 42), editorial letters (n = 4), survey of previous experiences (n = 11), books (n = 5), expanded abstracts (n = 1), case series (n = 19), case reports (n = 12), qualitative articles (n = 16), narrative review articles (n = 124), systematic reviews (n = 12), and meta-analyses (n = 7) were removed from the review process.
The included studies had been performed in 12 countries-i e, North America (n = 11), Europe (n = 4), South Asia (n = 3), Far East (n = 2), and Africa (n = 1). The included studies were conducted on 4041 subjects within the age range of 25 to 58 years and the male to female ratio of 1:1.4. The applied dosage of the sedative agents varied across studies. In this regard, etomidate, propofol, alfentanil, ketamine, midazolam, remifentanil, and fentanyl were administered at the dose ranges of 0.1 - 3.1 μg/kg, 0.02 - 2.5 mg/kg, 10 μg/kg, 0.2 - 2 mg/kg, 0.02 - 5.4 mg/kg, 0.5 - 10 mg/mL, and 0.1 - 21 µg/kg, respectively. Oxygen desaturation was observed in 0-28% of the patients sedated with various sedatives. In addition, emergence reaction and respiratory adverse events were observed in 36.2% and 76.7% of the patients, respectively. In different studies, sedation and recovery times were reported as 1.29 - 48.3 and 2.06 - 71.8 minutes, respectively (Table 1).
| Main Point | Authors (Year of Publication) Reference No. | Location | Sample Size | Age, yr; Mean (Range) | Female to Male Ratio | Total Dosage | Systolic Blood Pressure | Oxygen Desaturation | Emergence Reaction (Agitation) | Respiratory Adverse Events | Use of Bag Valve Mask | Sedation Time | Time to Recovery | Visual Analogue Scale; (Interquartile Range) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison of etomidate and propofol | Miner et al. (2007) (5) | USA | N: 214; E: 105; P: 109 | E: 36.9; P: 40.4; > 18 | E: 0.1 mg/kg followed by; 0.05 mg/kg every 3-5 min; P: 1 mg/kg bolus followed by 0.5 mg/kg every 3 min | E: 129.6 (64 - 178); P: 120.9 (60 - 158) | E: 9.5%; P: 9.1%; No difference | E: 34.3%; P: 42.2%; -7.9% (-20.9 to 5.1) | E: 3.8%; P: 4.6% | E: 8.8 min; P: 6.8 min | E: 18.3 mm; P: 16 m2 | |||
| Etomidate versus propofol | Desai et al. (2015) (27) | India | N: 60; E: 30; P: 30; | E: 38.80; P: 38.23 | 25/35 | E: 0.1 mg/kg; P: 1 mg/kg | E: 106.13 ± 15.2; P: 12.04; A difference was observed in 2 min | E: 16%; P: 33.3%; Difference | E: 435.7 sec; P: 659.1 sec; Difference | |||||
| Comparison of etomidate with propofol | Song et al. (2015) (28) | China | N: 80; E: 40; P: 40 | E: 55.8 ± 10.6; P: 52.4 ± 11.4 | 56/24 | E: 30 μg/kg; P: 0.3 mg/kg | No difference | E: 5.6 ± 0.8; P: 5.2 ± 0.9 | E: 14.5 ± 9.3; P: 15.2 ± 6.1 | |||||
| Propofol versus ketamine | Miner et al. (2010) (29) | USA | N: 97; P: 50; K: 47 | ≥ 18; P: 30; K: 34.5 | 49/48 | P: 1.46 mg/kg; K: 1 mg/kg | P: 127.5; K: 131.5 | P: 8%; K: 36.2% | P: 40%; K: 63.8%; No difference | P: 11 m; K: 10 m | P: 5 m; K: 14 m | No difference | ||
| Sedation level of moderate or deep procedural sedation using propofol | Miner et al. (2007) (30) | USA | N: 75; MS: 39; DS: 36 | ≥ 18 | MS: 0.88 mg/kg; DS: 0.98 mg/kg | MS: 11.4%; DS: 9.3%; No difference | MS: 15%; DS: 22%; No difference | MS: 7.97 min; DS: 8.19 M | MS: ; DS: | MS: 34.0 mm; DS: 28.8 mm | ||||
| Comparison of propofol with 1:1 and 4:1 mixtures of propofol and ketamine (ketofol) | Miner et al. (2015) (31) | USA | N: 271; P: 90; PK1: 85; PK2: 86 | P: 40; PK1: 39; PK2: 36; > 18 years | 143/128 | P: 10 mg/mL; PK1: 10 mg/mL (1:1); PK2: 5 mg propofol/mL and 5 mg ketamine/mL | P: 115; PK1: 122; PK2: 122 | P: 8; PK1: 21.2; PK2: 10 | P: 29%; PK1: 19%; PK2: 32% | P: 11%; PK1: 7%; PK2: 10% | P: 14 (5 to 25, 3 to 13); PK1: 16 (10 to 23, 5 to 231); PK2: 15 (11 to 18.5, 3 to 56) | |||
| Propofol versus alfentanil | Miner et al. (2017) (32) | USA | N: 108; A: 52; P: 56; | A: 32; P: 36 | 53/55 | A: 10 μg/kg; P: 1 mg/kg | A: 130; P: 128 | A: 37%; P: 25% | A: 0; P: 13% | A: 13; R: 13 | ||||
| Comparison of ketamine with and without midazolam | Sener et al. (2010) (33) | Turkey | N: 182; M: 90; NM: 92; | 30.5 (18 - 50) | 141/59 | 0.03 mg/kg IV midazolam; 1.5 mg/kg IV or 4 mg/kg IM ketamine | M: 0; NM: 0 | M: 8%; NM: 25%; CI: 6% - 28% | M: 0; NM: 0 | M: 35 (24 to 49); NM: 29 (24 to 37); CI: -2 to 8 | ||||
| Comparison of ketamine/propofol with midazolam/fentanyl | Nejati et al. (2011) (6) | Iran | N: 62; K/P: 31; M/F: 31; | Mean: 25 (20 - 37) | 25/6 | K/P-MF ratio: (1:1); 1.125 (0.75-1.5) mg; MF: 2 (2-3) mg; F: 0.04 (0.04-0.06) mg | Median; K/P: 4 (-30 to 33); MF: 0 (-40 to 10); 0.006 | KP: 3.2%; MF: -35.5% | KP: 29%; MF: 0 | Median (range); KP: 0 (-9 to 2); MF: -1 (-10 to 3); 0.58 | One patient in each group | K/P: 25.1 ± 13.8 m; MF: 26.1 ± 12.6 m (P = 0.77) | K/P: 18 ± 10.4 m; MF: 29.1 ± 10.2 | K/P: 0 (0 - 1); MF: 3 (1 - 6); (P < 0.001) |
| Comparison of midazolam with diazepam | Wright et al. (1992) (34) | USA | N: 69; D: 33; M: 36; | D: 32 ± 11; M: 30 ± 8 (18 - 60) | 33/36 | D: 2.5 mg/mL; M: 1 mg/mL | D: 132 ± 16; M: 131 ± 16; No difference | D: 18.9; M: 20.4 | ||||||
| Comparison of etomidate with midazolam | Gregory et al. (2005) (35) | USA | N: 45; E: 24; M: 21 | E: 43.3, F: 23; M: 53.4, F: 26; No difference | 32/13 | E: 0.10; M: 0.035 mg/kg | E: 1; M: 1 | E: 1; M: 0 | 2 patients | E: 15; M: 32 | ||||
| Propofol with and without midazolam | Rahman et al. (2015) (36) | Malaysia | N: 40; P: 20; PM: 20; | P: 40.6; PM: 35 | 31/9 | P: 1 mg/kg; PM: 0.1 mg/kg | P: 127.5; PM: 130 | P: 19.5%; PM: 18% | P: 29.3; PM: 71.8; Difference | P: ; PM: | ||||
| Propofol versus midazolam/fentanyl | Taylor et al. (2008) (37) | Australia | N: 86; P: 48; MF: 38 | P: 40.9; MF: 45.2; No difference | 60/26 | P: 1.8 mg/kg; MF: 0.1 mg/kg | Difference | P: 11/22.9%; MF: 6/15.8%; No difference | P: 6.8; MF: 28.5 | No difference | ||||
| Etomidate and midazolam | Burton et al. (2002) (38) | USA | N: 41; E: 19; M: 22 | E: 47 ± 23; E: 34 ± 14 | 31/10 | E: 0.1 mg/kg; M: 0.033 mg/kg | E: 0; M: 0 | E: 0; M: 0 | E: 10 m; M: 23 m; Difference | E: 16 mm; M: 31 mm; Difference | ||||
| Comparison of propofol with midazolam/ketamine | Uri et al. (2011) (39) | Israel | N: 60; P: 30; MK: 30 | P: 44; MK: 46.7 | 35/15 | P: 1.5 ± 0.4 mg/kg; M: 0.05 ± 0.02 mg/kg; K: 1.0 ± 0.3 mg/kg | Dropped significantly; following the propofol administration | P: 0; MK: 3.3% | P: 20%; MK: 10% | P: 20%; MK: 10% | P: 16.2 ± 3.8; MK: 41.6 ± 10.7; Difference | P: 7.8 ± 3.7; MK: 30.7 ± 10.1; Difference | No difference | |
| Comparison of ketamine plus low-dose midazolam with; midazolam/fentanyl | Cevik et al. (2013) (40) | Turkey | N: 61; K/M: 31; M/F: 30 | K/M: 34.1; M/F: 36.5 | 43/18 | K: 2 mg/kg; F: 2 μg/kg; M: 0.02 and 0.1 mg/kg | Increased in the KM; group and decreased in the MF group | K/M: 5.7% to 7%; M/F: 0 | K/M: 45.2; M/F: 76.7; difference | K/M: 0; M/F: 1 | K/M: 30; M/F: 32.5; No difference | Difference | ||
| Comparison of etomidate/remifentanil and propofol/remifentanil | Toklu et al. (2009) (41) | Turkey | N: 60; E/R: 30; P/R: 30; | E/R: 48 ± 11; P/R: 51 ± 11; 18 - 65 | 35/25 | E/R: 3.1 mg/kg; P/R: 2.5 mg/kg | Respiratory adverse events were more frequent in the; propofol group. | No difference | ||||||
| Ketamine/propofol (ketofol) versus propofol alone | Andolfatto et al. (2012) (42) | Canada | N: 284; PK: 142; P: 142 | PK: 48; P: 54 | 140/144 | PK: 0.75 mg/kg; P: 0.375 mg/kg | PK: 38%; P: 36% | PK: 3.5%; P: 11% | PK: 22%; P: 20% | PK: 2%; P: 1% | PK: 7 m; P: 7 m | PK: 8 m; P: 6 m | ||
| Comparison of propofol with midazolam | Hisamuddin (2010) (36, 43) | Malaysia | N: 40; P: 20; M: 20 | 37.8 | 31/9 | P: 1 mg/kg; M: 0.1 mg/kg | P: 122.5; M: 120 | P: 19.5%; M: 18% | P: 59.06; M: 30.33; Difference | |||||
| Midazolam versus propofol | Holger et al. (2005) (44) | USA | N: 40; P: 20; M: 20 | 18 - 65 | P: 0.96 mg/kg; M: 5.4 mg; | P: 35; M: 24; Median | Median; P: 36; M: 52; (P = 0.007) | A mean; difference of 20 min in the time of recovery | P: 85; M: 90; No difference | |||||
| Comparison of remifentanil/propofol with morphine/midazolam | Dunn et al. (2011) (45) | UK | N: 40; RP: 20; MM: 20 | 18 - 65 | 11/29 | Remifentanil: 10 mg/mL; Propofol: 5 mg/mL; Morphine and midazolam: 1 mg/mL (10 mg in 10 mL of 0.9% w/v sodium chloride) | RP: 15 m; NM: 45; Difference | No difference | ||||||
| Comparison of propofol with midazolam | Hatamabadi et al. (2015) (46) | Iran | N: 48; MF: 29; PF: 19; | 31.9 (18 - 60) | 48/0 | M: 2 mg/kg; P: 0.5 mg/kg) | MF: 48.3; PF: 15.4 | |||||||
| Etomidate versus midazolam | Chan et al. (2008) (47) | South Korea | N: 78; E: 36; M: 42 | E: 57; P: 56 | 32/46 | E: 0.1 mg/kg; M: 0.05 mg/kg | E: 9.1 m; M: 11 m; No difference | E: 278.9 (596.3); M: 169.6 (226.7); No difference | E: 27.7 (30.4); M: 35.4 (30.7); No difference | |||||
| Ketamine versus fentanyl | Messenger et al. (2008) (48) | Canada | N: 63; PK: 32; PF: 31 | (14 - 65) | 31/32 | PK: 0.3 mg/kg; PF: 1.5 μg/kg | PK: 17.7 ± 141.9; PF: 19.4 ± 137.6 | |||||||
| Etomidate versus ketamine | Jabre et al. (2009) (49) | France | N: 469; E: 234; K: 235 | E: 57; K: 59 | 280/189 | E: 0.3 mg/kg; K: 2 mg/kg | E: 132; K: 128 | E: 10%; K: 9% | E: 16%; K: 17% | |||||
| Remifentanil pretreatment reduces myoclonus after etomidate | Kelsaka et al. (2006) (50) | Turkey | N: 60; R: 30; Placebo: 30 | P: 54; K: 48 | 32/28 | R: 1 μg/kg | K: 38%; P: 36% | K: 5%; P: 15% | K: 43%; P: 46% | K: 3%; P: 1% | K: 7 min; P: 7 min | K: 8 min; P: 6 min | ||
| Propofol versus ketofol | Ferguson et al. (2016) (51) | Australia | N: 573; P: 281; K: 292 | P: 46; K: 50 | 283/290 | P: 0.02 μg/kg; K: 0.02 μg/kg | P: 20%; K: 12% | P: 17%; K: 19% | P: 3%; K: 1% | P: 24 m; K: 33 m | Shorter recovery time with propofol | |||
| Morphine versus fentanyl | Galinski et al. (2007) (52) | France | N: 54; M: 26; F: 28 | M: 40; F: 45 | 45/9 | M: 0.1 mg/kg; F: 0.1 mg/kg | M: 129; F: 131; No difference | M: 19%; F: 16%; No difference | M: 83; F: 77; No difference | |||||
| Fentanyl, propofol, midazolam, and ketamine versus propofol/fentanyl | Amini et al. (2018) (53) | Iran | N: 125; PFM: 63; PF: 62 | PFMK: 37.2 ± 13.2; PF: 38.2 ± 15.6 | 91/34 | PFMK: 0.5 mg/kg; 0.5 - 1 µg/kg; 0.1 - 0.02 mg/kg; 0.2 - 0.25 mg/kg; PF: 1 mg/kg; 1 mg/kg | PFM: 123.4 ± 12; PF: 124.8 ± 12.7 | PFMK: 6.48 ± 1.84; PF: 5.02 ± 4.47; Difference | ||||||
| Fentanyl/midazolam versus remifentanil | Gharavifard et al. (2016) (54) | Iran | N: 96; FM: 48; R: 48; | FM: 39.8 ± 9.9; R: 39.7 ± 10.3; (18 - 64) | 83/16 | F: 1.5 µg/kg; M: 0.1 mg/kg; R: 1 µg/kg | FM: 1.2%; R: 0; No difference | FM: 4.6 ± 1.8; R: 2.5 ± 1.6; Difference | FM: 70.3 ± 10.8; R: 76.6 ± 11.9; No difference | |||||
| Propofol/fentanyl versus remifentanil | Kasmaee et al. (2019) (55) | Iran | N: 64; PF: 32; R: 32; | PF: 35.43 ± 14.25; R: 34.28 ± 10.84 | 16/48 | PF: 0.5 mg/kg; R: 0.5 mg/kg | PF: 0; R: 25%; Difference | PF: 31.3%; R: 9.4% | PF: 1.29 ± 0.48; R: 1.96 ± 1.77; Difference | PF: 2.06 ± 1; R: 5.43 ± 4.9; Difference | PF: 1.15 ± 0.36; R: 1.59 ± 0.79; Difference | |||
| Propofol/fentanyl versus ketofol | Aminiahidashti (2018) (19) | Iran | N: 136; PF: 70; PK: 66 | PF: 33.77; PK: 31.71 | 88/48 | F: 1 mg/kg; P: 0.5 mg/kg; K: 1 mg/kg | PF: 124.03; PK: 126.67; No difference | PF: 15.7%; PK: 13.7%; No Difference | PF: 22; PK: 24; No difference | PF: 4; PK: 5; Difference | ||||
| Fentanyl/propofol versus ketofol | Khajavi et al. (2013) (56) | Iran | N: 60; PK: 30; PF: 30; | PK: 55.9 ± 15; PF: 51.6 ± 21; | 34/26 | P: 0.5 mg/kg; K: 0.5 mg/kg; F: 1 µg/kg; | No difference | PK: 21.8 ± 9.7; PF: 23.3 ± 7.7 | PK: 45.3 ± 8.4; PF: 50.6 ± 6.2 | |||||
| Ketofol versus fentanyl/propofol | Hasanein et al. (2013) (57) | Egypt | N: 20; PK: 100; PF: 100 | PK: 57.67 ± 13.3; PF: 56.93 ± 11.9 | 99/101 | PK: 10 mg/mL; PF: 1.5 μg/kg | PK: 0%; PF: 7%; Difference | PK: 2%; PF: 0; No difference | PK: 2%; PF: 10%; Difference | PK: 9.43 ± 1.23; PF: 11.19 ± 2.59 min; Difference |
Abbreviations: A, alfentanil; D, diazepam; DS, deep sedation; E, etomidate; E/R, remifentanil; K, ketamine; K/P, ketaminel propofol; M, midazolam; M/F, midazolam/fentanyl; MS, moderate sedation; NM, non-midazolam; P, propofol; PK1, propofol with ketamine (1:1); PK2, propofol with ketamine (4:1)
3.3. Quality and Risk of Bias Assessment
In the present systematic review, the majority of the studies had a low risk of bias; in addition, no article was removed due to low quality. The included studies had some clinical heterogeneities varying from low to high levels in sample size, indications for sedation, consumed medication, dosage, procedure, and reason for referring to ED. All studies were performed on adults transferred to ED. Figure 2 presents the risk of bias in the included trials.
4. Discussion
4.1. Propofol Versus Ketamine or Ketofol
Generally, the efficacy of propofol is similar to that of other medications for PSA in EDs (58). Propofol may be applied alone or in combination under emergency conditions. Synergistic effects of ketamine in combination with propofol on sedation and analgesia were confirmed in a systematic review (59). Ketamine is different from other sedative agents since it lacks the feature of the dose-response continuum to progressive titration. This medication can produce analgesic and sedative effects below some critical dosage thresholds (e g, 1 - 1.5 mg/kg IV or 3 - 4 mg/kg IM) (33).
The literature search revealed only one study comparing propofol and ketamine (29) and three studies comparing propofol and ketofol for procedural sedation in ED (31, 42, 51). In this regard, Miner et al., showed that the subclinical respiratory depression was lower in the propofol group than in the ketamine group. However, they observed no difference in the median time of procedures between the ketamine and propofol groups (11 vs. 10 minutes). However, the median time to return to the baseline mental status after the procedure was longer in the ketamine group than that of the propofol group (14 vs. 5 minutes). In the mentioned study, pain during the procedure was reported in 6% and 2.1% of the patients in the propofol and ketamine groups, respectively. Moreover, recovery agitation was observed in 8% and 36.2% of the patients in the propofol and ketamine groups, respectively (29).
High IV doses and coadministration of anticholinergics or benzodiazepines were the risk factors of respiratory adverse events after the administration of ketamine (60). Another effect of ketamine was the incidence of emergence delirium, observed more frequently among the adults admitted to EDs than children (61). This adverse event had an incidence of 13% in adults (62). Age, dosage, gender, psychological susceptibility, and concurrent drug usage were the main factors affecting the incidence of emergence reactions (63).
Ketofol is probably accompanied by a lower rate of unpleasant emergence phenomena than ketamine (64). Ketamine can lead to the rise of thalamic sensory output and arousal, thereby controlling the sedative effect of propofol. It could be the result of the dose-dependent interaction of ketamine with propofol (65). In a study performed by Hasanein et al., the incidence of emergence agitation and postoperative nausea and vomiting was higher in the ketofol group than in the fentanyl/propofol group (57).
In the study by Miner et al., no difference was observed in the frequency of adverse airway or respiratory events among the patients receiving propofol alone and the ones receiving propofol plus ketamine in the ratios of 1:1 and 4:1. Moreover, they reported no intubation or aspiration in any group. The time spent at each level of sedation and reported pain scores were similar among the three groups. In addition, they reported a higher frequency of recovery agitation in the propofol/ketofol (1:1) group (31).
The incidence of hypoxia secondary to propofol usage is reported in about 5% of patients (66, 67). In a study performed by Akin et al., the risk of respiratory depression and the need for repeating medication administration decreased after using a low-dose of ketamine-propofol combination. Moreover, the mean arterial pressure was controlled by using the mentioned combination (68, 69). In addition, Goh et al., reported that the administration of ketofol resulted in the minimization of apnea and optimization of hemodynamics (70).
4.2. Propofol/Fentanyl Versus Ketofol
In the present literature review, there were three studies comparing propofol/fentanyl with ketofol for PAS in patients referring to EDs (19, 56, 57). In one of them, Aminiahidashti et al., reported that propofol/fentanyl resulted in deeper sedation, better analgesic effects, and higher pain reduction compared to propofol/ketamine. Moreover, no considerable difference was observed between the two groups in terms of respiratory adverse events (19). In another study, Khajavi et al., compared the effect of a bolus IV injection of ketofol with that of fentanyl/propofol combination during the colonoscopy procedure and, in this study, similar to that of Aminiahidashti et al., the respiratory adverse events were not different between the patients receiving propofol/fentanyl and those subjected to ketofol (56). Generally, psychomimetic reactions may occur by a large-dose ketamine injection (56). In the study by Khajavi et al., the incidence of psychomimetic reactions was estimated at 7.5% (56). Low psychomimetic reactions in the mentioned study might be due to the injection of midazolam or a combination of ketamine and propofol to all patients.
Generally, the determination of the appropriate proportion of propofol/ketamine in preparing ketofol infusion is a challenging issue. Based on the literature, the ketamine/propofol combination is administered in various ratios from 1:1 to 1:5 (71, 72). Although all combinations are accompanied by hemodynamic stability, a higher proportion of ketamine results in prolonged discharge. In the study by Akin et al., the mean arterial pressure was better maintained at the ketamine/propofol ratio of 1:3 compared to propofol monotherapy (69). In another study, the comparison of propofol alone with a 3:1 propofol/ketamine combination showed that the administration of propofol/ketamine combination resulted in no desaturation case. However, the risk of respiratory depression and the need for more medication decreased with the addition of a low dose of ketamine to propofol.
4.3. Propofol or Ketofol Versus Midazolam
A total of eight included studies compared propofol or ketofol with midazolam or a combination of midazolam/fentanyl. In this regard, in a study by Rahman et al., propofol was compared with midazolam for PSA in ED. Their results showed no significant adverse events during and after the procedures. Therefore, they considered both propofol and midazolam as safe and effective agents for PSA (36). Their results were consistent with those of other similar studies (43, 46). Hatamabadi et al., reported that the rates of sedation induction and recovery of consciousness were higher in patients receiving propofol compared to the ones in the midazolam group. Furthermore, a shorter PSA time was reported in the propofol group in comparison with the midazolam group (46).
In a similar study, it was concluded that propofol requires less monitoring and is more cost-effective than midazolam. Moreover, the results indicated that propofol administration led to high physician satisfaction compared to midazolam usage (44). The combination of propofol and midazolam can be titrated to achieve a moderate level of anesthesia. However, given the low analgesic effect of this combination results in a high sense of pain, it is associated with low patient satisfaction (73).
In a study by Uri et al., comparing propofol with midazolam/ketamine, it was demonstrated that the recovery and sedation times were shorter in the propofol group than the midazolam/ketamine group. In addition, the respiratory and hemodynamic adverse events were higher in the propofol group compared to those of the midazolam/ketamine group (20% vs. 10%) (39). Taylor et al., showed that the patients in the propofol group had a shorter time to first wakening, better recovery of consciousness, easier shoulder reduction, and fewer reduction attempts than the ones in the midazolam/fentanyl group (37).
4.4. Propofol Versus Alfentanil
Among the included studies, there was a randomized clinical trial of comparing alfentanil with propofol. Based on the obtained results, the airway or respiratory adverse events were similar in the two groups, and no serious adverse events were reported in any of the groups (32).
4.5. Propofol Versus Etomidate
Hypotension and respiratory depression may occur after the administration of propofol. However, due to the low risk of hypotension with etomidate administration, it is considered as a safe sedative agent for hemodynamically unstable patients. Three of the reviewed studies compared etomidate with propofol for PSA in adults admitted to ED (5, 27, 28). In this respect, Miner et al., reported no clinically significant complications for etomidate and propofol. In the mentioned study, the time to return to the baseline mental status was longer in the patients receiving etomidate compared to the ones in the propofol group. In addition, a higher rate of subclinical respiratory depression was reported in the adults undergoing procedural sedation with etomidate, compared to the patients undergoing PSA with propofol.
The results of the mentioned study revealed no difference between etomidate and propofol groups in terms of sedation, hypoxia, apnea, and clinical events related to respiratory depression. Furthermore, myoclonus was higher in the etomidate group than in the propofol group (20% vs. 1.8%). There was also no difference between the two groups in terms of the increased supplemental oxygen, use of a bag valve mask, airway repositioning, and stimulation to induce breathing (5). In the mentioned study, the mean total dose of etomidate was 0.26 mg/kg, which was in line with the value reported in a similar study (38). Nonetheless, the mean initial dose of etomidate was higher in the study by Miner et al. They found no significant difference in the respiratory depression of patients undergoing deep and moderate sedation (30). Other studies also noted a decrease in systolic blood pressure following the administration of different doses of propofol (74, 75).
The administered dosage in the study by Miner et al., was similar to those reported in other studies (66, 76, 77). Given the different responses of patients to the same dose of medication, the sedatives are titrated until the patient seems adequately sedated (30).
4.6. Etomidate Versus Midazolam and Ketamine
Three studies compared etomidate with midazolam for PAS in patients referring to the EDs (35, 38, 47). Chan et al., found no differences between the etomidate and midazolam groups in terms of pain score, adverse effects, total procedure time, and total length of hospital stay. However, the etomidate group had a shorter mean time for the onset of action compared to the midazolam group (47). The findings obtained by Chan et al., were consistent with the results of a similar study (38). Gregory et al., showed that the etomidate group had a shorter sedation time as compared to the midazolam group. They also reported a reduction in the recovery time of the etomidate group compared with that of the midazolam group (35). There was only one study comparing the effectiveness of etomidate with that of ketamine for endotracheal intubation in critically ill patients. The results suggested ketamine as a safe and valuable alternative to etomidate (49).
4.7. Etomidate and Remifentanil
Two studies conducted in Turkey compared the efficacy of etomidate with that of remifentanil for PSA among adults referring to EDs. In a study by Toklu et al., the comparison of etomidate/remifentanil with propofol/remifentanil revealed that the mean arterial pressure was lower in the propofol group than the etomidate group. Furthermore, heart rate, SpO2, respiratory rate, and apnea were significantly similar in the two groups. The groups also had no significant difference in the Ramsay sedation score (41).
In another study, no significant difference was observed in apnea incidence between the patients treated with etomidate and propofol for PSA (77). However, Miner et al., observed subclinical respiratory depression in 34% and 42% of the patients in the etomidate and propofol groups, respectively. They also reported myoclonus in 20% and 1.8% of the patients in the etomidate and propofol groups, respectively (5). This condition was reported in 15% of the patients receiving propofol in a study by Ruth et al. (78). The results of the study by Miner et al., on the rate of subclinical respiratory depression and hypoxia in patients receiving propofol were similar to those of other studies (22, 76).
There was evidence indicating a higher but insignificant arterial blood pressure in the etomidate group compared to the propofol group (78, 79). Nausea and vomiting are the common side effects of etomidate, with an incidence of 50% when administered in repeated doses (80).
4.8. Midazolam Versus Ketamine or Diazepam
Sener et al., observed no difference between patients receiving IV and IM injections of ketamine/midazolam combinations in terms of sedation time (33). In the mentioned study, recovery agitation was observed in 33% of the patients, which was significantly lower in patients receiving midazolam. Accordingly, they concluded that the addition of midazolam to ketamine can decrease the incidence of recovery agitation (33). Similarly, in a study by Sener et al., midazolam could reduce the incidence of recovery agitation after the administration of ketamine for PSA. They also indicated that the incidence of adverse events was similar in both the groups subjected to IV and IM administration (33).
Cevik et al., compared a midazolam/fentanyl combination with a low-dose combination of ketamine and midazolam and indicated the priority of ketamine/midazolam combination over midazolam/fentanyl due to lower hypoxia, duration of hypoxia, and pain score (40).
4.9. Propofol/Fentanyl Versus Remifentanil
The efficacy of remifentanil and propofol/fentanyl combination was assessed in two of the reviewed studies. Based on the study by Kasmaee et al., propofol/fentanyl combination was as effective as remifentanil in pain management in patients with shoulder dislocation. Moreover, the onset of action and recovery times were shorter in the propofol/fentanyl group compared to the remifentanil group. However, the propofol/fentanyl group showed a lower success rate in muscle relaxation and a higher rate of apnea (55). These findings were confirmed by similar studies (81-83). In the study by Kasmaee et al., agitation and apnea were observed in 25% and 9.4% of the patients in the propofol/fentanyl group, respectively; the frequency of apnea was 32% in the remifentanil group (55).
4.10. Remifentanil Versus Midazolam/Fentanyl or Morphine/Midazolam
There were few studies on the administration of remifentanil for sedation to adults referring to EDs. A study by Dunn et al., reported that remifentanil could provide profound analgesia and quick recovery in adults (45). Gharavifard et al., estimated the failure rates of 31.3% and 2.1% in the midazolam/fentanyl and remifentanil groups, respectively. Moreover, they demonstrated that the subjects sedated by remifentanil had a shorter procedure and greater pain reduction. The frequency of respiratory and non-respiratory adverse events was not significantly different between the two groups. Patient satisfaction was higher in the remifentanil group than in the midazolam/fentanyl group (54), which was consistent with the results obtained by Dunn et al. In the mentioned study, the median recovery times were 15 and 45 minutes in the remifentanil/propofol combination and morphine/midazolam groups, respectively. They also found no difference between the two groups in terms of the pain reduction conditions and pain/distress scores (81). Both propofol and remifentanil provide excellent sedation and analgesia for the reduction of anterior glenohumeral dislocation (45, 81).
4.11. Remifentanil and Fentanyl/Midazolam
In a study by Kelsaka et al., the incidence of myoclonus was estimated at 6.7% and 70% in the remifentanil and control groups, respectively. They observed a reduction in the incidence of myoclonus with etomidate administration following the injection of 1 mg/kg remifentanil (50). The length of the procedure, procedural features (anxiolysis vs. immobility), and the need for prolonged sedation should be noted before choosing a sedative agent for PSA; therefore, it is required to perform a risk-benefit analysis before PSA induction (54).
5. Conclusion
Information about the available medications, as well as their proper dosage and side effects, is a matter of paramount importance for the anesthesiologists. The main concern of emergency medicine specialists is to find an appropriate, safe, and fast-acting medication with the fewest side effects for PSA. Various combinations of medications are used for PSA depending on the hospital protocols and policies; however, there is still a controversy over the best choice. Based on the results of the reviewed studies, the most common medication used for PSA is propofol due to its short sedation time, rapid recovery of consciousness, and few side effects.