1. Background
Cirrhosis, the consequence of chronic liver damage, is associated with cardiovascular abnormalities, including an increase in cardiac output, a decrease in arterial pressure, and total peripheral resistance. An increased systolic, diastolic function and electrophysiological abnormality such as QT prolongation is reported in cirrhotic patients (1, 2). Recent developments in techniques and precise measurement of cardiovascular variables could determine the anomalies in cirrhosis. “Cirrhotic cardiomyopathy” has been clinically defined by an expert group in 2006 as increased or normal left ventricular systolic contractility at rest, decreased systolic contraction, or diastolic relaxation in stress conditions such as pharmacologic, physiologic and surgical stresses, and cardiac electrical abnormalities (2).
Cardiac electrophysiological abnormalities have not yet been completely understood in cirrhotic patients. Although common physiological modifications such as autonomic neuropathy have been considered as a probable cause of QTc prolongation (3), the exact cause in cirrhosis has not been clarified. It commonly occurs in chronic liver disease and affects ventricular repolarization and QT interval. The association of QTc prolongation and cardiac arrhythmia with enhanced mortality rate could be caused by cardiac disease, electrolyte abnormalities, and many regular medicines (cisapride, phenothiazines, erythromycin, trimethprime, sulfamethoazole, tricyclic anti-depressants, and quinidine) (4, 5).
Earlier studies had reported the QT prolongation in alcoholic cirrhotic patients. However, subsequent studies have shown QT prolongation in almost all causes of cirrhosis (viral and non-viral). Although the QT prolongation could be affected by the severity of cirrhosis (Child-Pugh score) and plasma norepinephrine (a marker of sympathetic nervous system activity) concentration, but it also occurs in well-compensated cirrhosis (3). Commonly, sympathetic activity leads to decreased QT interval, but in cirrhosis, it results in QT prolongation (6). Moreover, QT prolongation can be associated with ventricular arrhythmias such as Torsade de Pointes (3) and altered repolarization in ventricles, which is accordingly a part of cirrhotic cardiomyopathy (1).
2. Objectives
The present study was designed to evaluate the diurnal QTc the changes in non-alcoholic cirrhosis compared to the healthy controls, and its relationship to cardiovascular factors such as heart rate and blood pressure to provide a more accurate evaluation of cardiac electrophysiologic abnormalities.
3. Methods
3.1. Study Population
The present case-control study consisted of 15 (7 females, 8 males) known cases of non-alcoholic cirrhosis as the study group and 15 sex-and-age-matched healthy subjects as the control group. The exclusion criteria were any history of diabetes, renal, cardiovascular and neoplastic diseases and treatment with calcium channel blockers, digoxin, antiarrhythmic agents, vasopressin, trimethoprim/sulfamethoxazole, tricyclic anti-depressants (TCA) and other medications that prolong QT interval, such as erythromycin, pentomidine, phenothiazines and cisapride or encephalopathy above grade I.
Patients were categorized based on Child-Turcotte-Pugh classification through laboratory parameters (serum albumin, bilirubin, prothrombin time, liver enzymes, BUN levels, creatinine, serum sodium, potassium, and calcium), endoscopy results (eosophageal varices), encephalopathy and the report of liver transient elastography.
A liver stiffness measurement was performed using the FibroScan® 502 machine (EchoSense, Paris, France, 5 MHz). According to the manufacturer’s guidelines, the M (medium) probe was used for subjects with thoracic perimeter less than 110 cm and the XL (X-large) probe for 110 cm and above. While patients were lying in the dorsal decubitus position with maximal abduction of the right arm, the probe was placed on their skin, overlying the right lobe of the liver through the intercostal spaces. At least 10 measurements were done for each patient, and the median value was recorded. Values were considered valid if the interquartile range (IQR) were less than 30% of the median reading, and the success rate was at least 60%. Cases were asked to have 3 hours of fasting, then Fibroscan was performed by a trained medical doctor. If there were any ascites, metal tools in the body, pregnancy, or morbid obesity, liver stiffness measurement was canceled regards to the manufacturer’s instructions.
According to this classification, six patients (40%) were classified as class A, six (40%) as class B, and three patients (20%) as class C.
3.2. QT and QTc Measurements
We recorded twenty-four-hour electrocardiogram (ECG) Holter-monitoring using a three-channel Holter recorder (Cardiolight 9.2a modest, Germany). Beta-blocker treatment was withdrawn for 24 - 48 hours during Holter-monitoring due to the effect of beta-blockers on QT interval. Since QT interval was dependent on heart rate, all intervals were corrected for heart rate according to assess diurnal variation, a day was divided to four-hour intervals including early morning (4 - 5 AM), morning (8 - 9 AM), noon (12 - 13 PM), afternoon (16 - 17 PM), night (20 - 21 PM) and midnight (0 - 1 AM). The variables were determined for each part.
Another parameter evaluated in this study was QT dispersion (QTdisp = QTmax - QTmin) and corrected QT interval (QTc disp = QTc max - QTc min) that indicate heterogeneity during ventricular repolarization. Blood pressure was measured during twenty-four hours. Furthermore, mean arterial blood pressure was calculated (mean arterial blood pressure = 2/3 diastolic blood pressure + 1/3 systolic blood pressure).
3.3. Statistical Analysis
Data were tested for normality distribution via the Kolmogorov-Smirnov test. Independent t-test and ANOVA were used for normally distributed variables; their nonparametric equivalents were employed for those who were not normally distributed. The results are expressed as percentages and means ± SD. P < 0.005 was considered significant.
3.4. Ethical Considerations
The study protocol was approved by the local ethical committee of Golestan University of Medical Sciences (code: goums.104391040404). All patients signed the informed consent after we explained the project and answered their questions.
4. Results
We selected 45 from the non-alcoholic cirrhotic patents15 patients (7 females and 8 men) were finally fulfilled the eligibility criteria and included in our study. The mean (SD) age was 55.93 (2.2) years. A group of age and sex-matched healthy people were recruited as the control group (mean (SD) age of 52.47 (1.08) years).
Cirrhosis occurred due to hepatitis B (n = 7), cryptogenic hepatitis (n = 5), hepatitis C (n = 1), and autoimmune hepatitis (n = 2). Fibroscan results confirmed severe fibrosis in 12 patients and intermediate results in 2, which was confirmed considering clinical symptoms and laboratory ultrasonography data.
Ascites has been seen in 7 patients. Propranolol was held for 24 - 48 hours, if possible. None of the patients took drugs with the side effect of QT-prolongation. Serum levels of sodium, potassium, and calcium were in normal range. Regarding to Child-Turcotte-Pugh classification, 40% of patients classified as child-A (n = 6), 40% as child-B (n = 6), and 205 as child-C (n = 3). Table 1 shows other data.
| Healthy Controls (N = 15) | Cirrhosis (N = 15) | ||||
|---|---|---|---|---|---|
| A (N = 6) | B (N = 6) | C (N = 3) | Total (N = 15) | ||
| Age, y | 52.47 ± 1.08 | 55.17 ± 3.0 | 59.67 ± 2.6 | 58.0 ± 5.1 | 55.93 ± 2.2 |
| Sex (M/F) | 8/7 | 4/2 | 2/4 | 2/1 | 8/7 |
| Ascitis | 0 | 0 | 5 | 2 | 7 |
| Diuretics (yes/no) | 0 | 1 | 4 | 3 | 8 |
| Propranolol (yes/no) | 0 | 2 | 4 | 1 | 7 |
| Serum K, mmol/L | 4.1 ± 0.08 | 4.3 ± 1.0 | 4.5 ± 0.1 | 5.1 ± 0.1 | 4.5 ± 0.1b |
| Serum Na, mmol/L | 141.7 ± 0.8 | 144.0 ± 2.6 | 144.9 ± 2.3 | 141.3 ± 3.2 | 143.8 ± 1.5 |
| Serum C, mmol/L | 8.7 ± 0.1 | 8.7 ± 0.3 | 9.0 ± 0.1 | 8.5 ± 0.5 | 8.7 ± 0.3 |
| Serum Cr, mmol/L | 0.8 ± 0.03 | 0.82 ± 0.4 | 0.85 ± 0.1 | 0.92 ± 0.1 | 0.85 ± 0.06 |
| Serum BUN, mmol/L | 17.8 ± 2.4 | 36.5 ± 5.3 | 30.6 ± 4.3 | 33.0 ± 2.6 | 33.47 ± 2.7b |
| Serum albumin, g/dL | 4.4 ± 0.1 | 3.3 ± 0.1 | 3.6 ± 0.2 | 2.5 ± 0.03 | 3.3 ± 0.1b |
| Serum bilirubin, mg/dL | 0.7 ± 0.05 | 1.2 ± 0.08 | 1.68 ± 0.2 | 2.6 ± 0.1 | 1.68 ± 0.1b |
| Serum alkaline phosphatase, IU/L | 153.3 ± 6.2 | 306.3 ± 82.32 | 227.5 ± 24.9 | 452.7 ± 145.6 | 304.1 ± 46.3b |
| Serum ALT, IU/L | 19.1 ± 2.0 | 42.0 ± 12.18 | 28.1 ± 4.5 | 53.3 ± 28.9 | 38.73 ± 7.3b |
| Serum AST, IU/L | 20.7 ± 1.5 | 44.8 ± 7.8 | 46.8 ± 8.0 | 73.3 ± 39.9 | 51.33 ± 8.5b |
aValues are expressed as mean ± SD.
bData considered different at the level of P < 0.05.
4.1. QT Interval
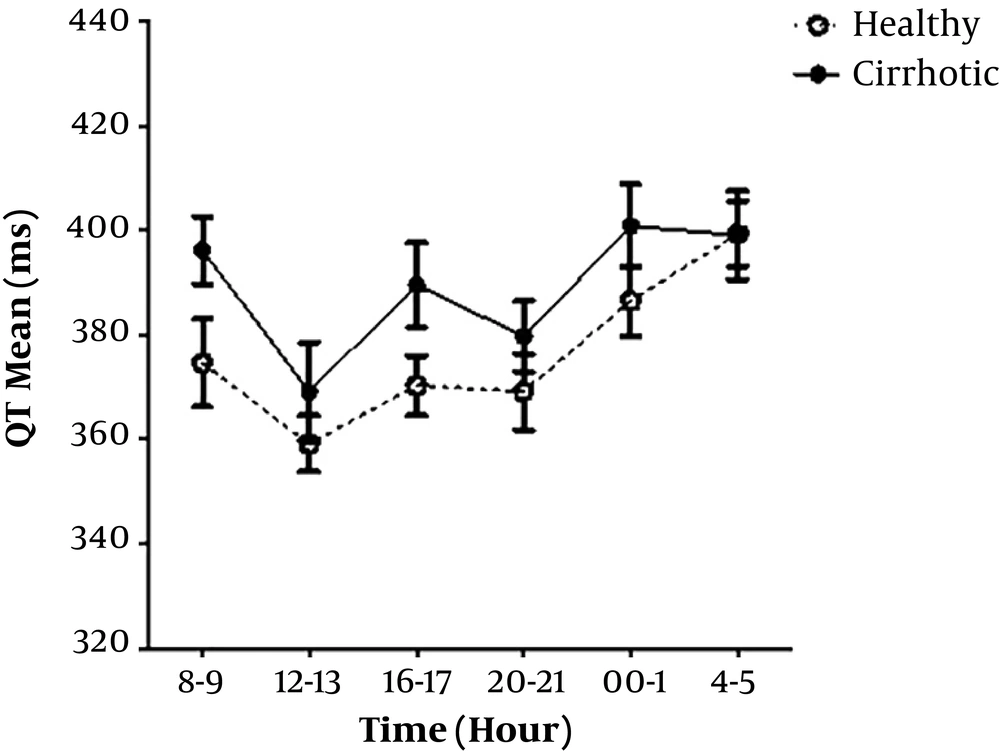
Consecutive recording of QT intervals was revealed to a longer mean 24-h QT interval in the cirrhotic group compared to the control group (P = 0.78, not significant), although the difference of QTc interval was significant between two groups (P = 0.03) (Table 2).
| Cirrhosis | Control Group | P Value | |
|---|---|---|---|
| QT interval, ms | 376.6 ± 5.4 | 392.1 ± 8.0 | 0.78 |
| QTc interval, ms | 401.7 ± 4.5 | 438 ± 7.1 | 0.03 |
aValues are expressed as mean ± SD.
In cirrhotic group, higher Child-Pugh classifications showed higher QT interval (not significantly) (Figure 1).
4.2. Corrected QT Interval (QTc)
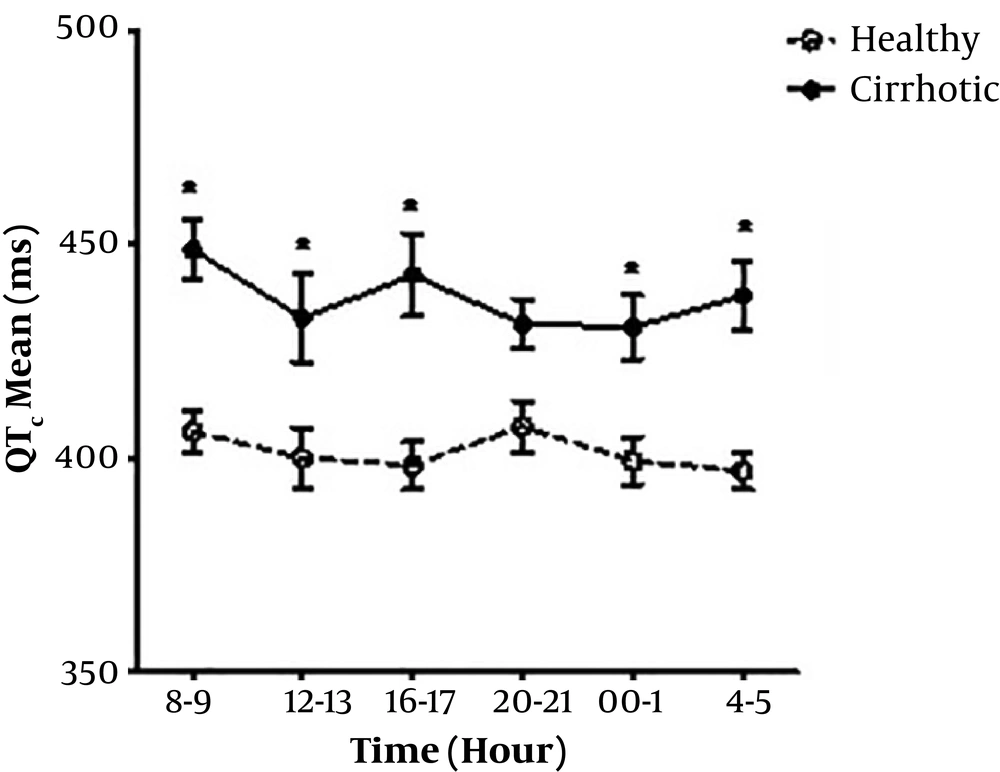
Mean QTc value was not significantly different between cirrhotic men and women (439.7 ms V.S. 436.1 ms). QTc prolongation was found in seven patients (4 males, 3 females) out of 15, but no QTc prolongation was observed in the control group. Comparing the mean and diurnal variability of QTc was found significantly (P = 0.03) higher in the cirrhotic group than the controls (Table 1). Mean QTc value was longer in healthy female subjects than male, although it was not significant (408.3 ms V.S. 395.5 ms, P = 0.85) (Figure 2).
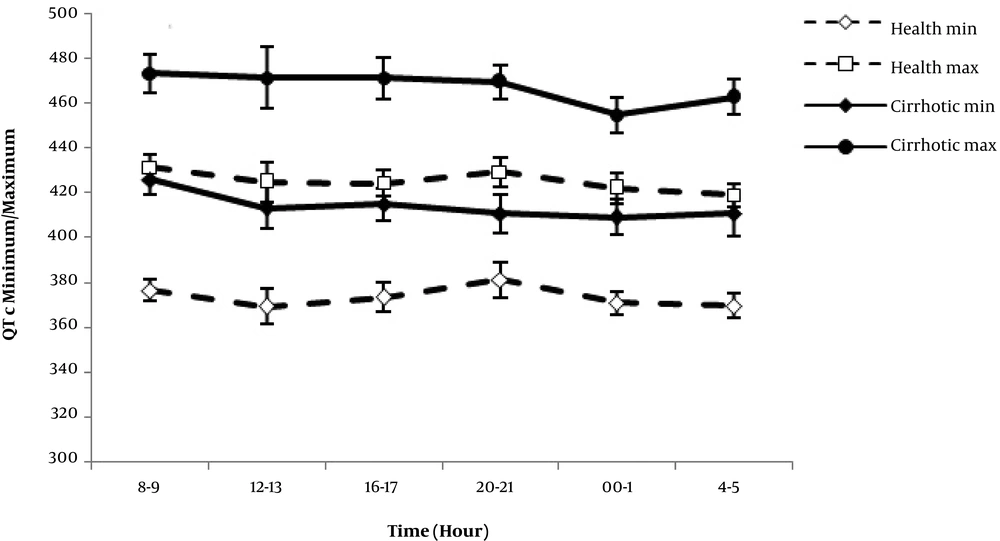
The maximum and minimum QTc were significantly longer in cirrhotic patients. Neither group showed a significant difference between diurnal QTc max and QTc min variability patterns at different times, whereas QTc max and QTc min measurements were significantly greater in the cirrhotic group over 24 hours (P = 0.00) (Figure 3).
Comparison of QTc max, QTc min, and QTc disp in cirrhotic and control group showed a significant difference (P value = 0.03). The greatest value of QTdisp was found in the child C group of cirrhotics.
4.3. Cardiovascular Factors and the QT Interval
The regression analysis showed that QT was correlated with QTC in both groups. The squared correlation coefficient was 34% and 30% in the cirrhotic group and the healthy control group, respectively. QT interval was positively correlated with QTdis over 24-hours in cirrhotics and the healthy control group and R2 score were 25% and 13% in cirrhotic and the healthy control group, respectively. The heart rate corrected QT, QTc and QTc disp were negatively correlated in healthy controls (R2 = 2%). By contrast, they were positively correlated in the cirrhotic group.
4.4. Heart Rate Variations
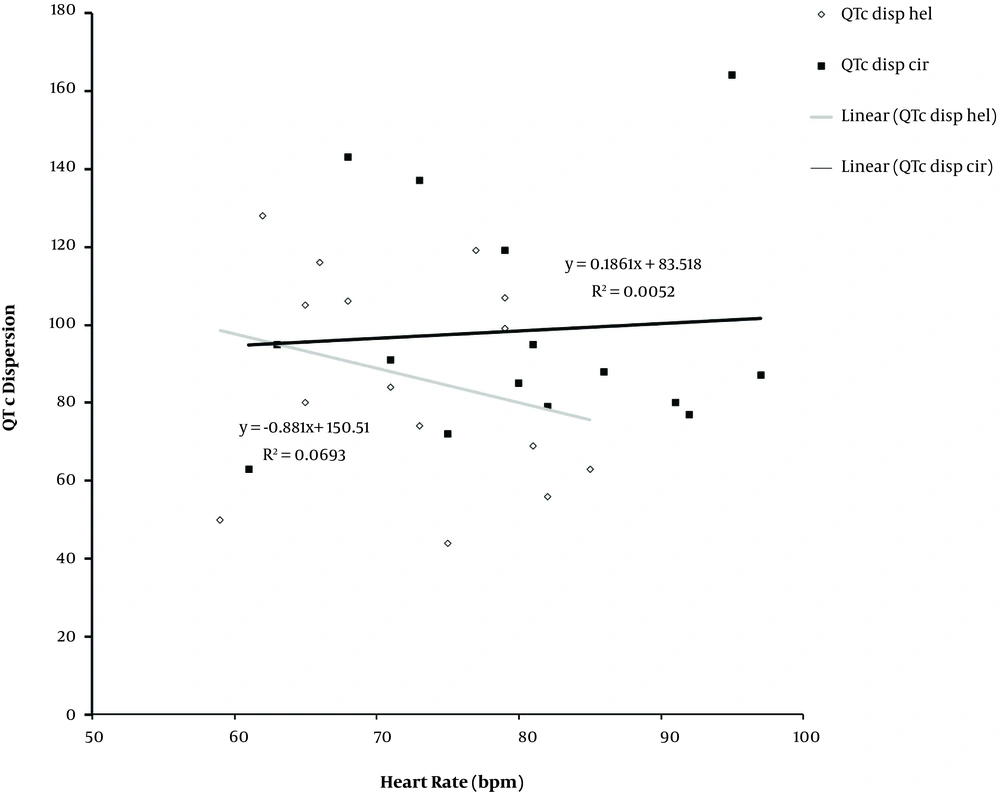
Mean heart rate over 24-hours were 79.6 ± 2.9 bpm and 72.47 ± 2.0 bpm in cirrhotic group and controls, respectively (P value = 0.05). Heart rate variability pattern was slightly different between the two groups, and a significant difference was seen in the early morning. Heart rate values over 24-hours were greater in cirrhotics compared to the healthy subjects. Linear regression graph of heart rate and QTdisp showed a negative correlation in both groups, R2 score values were 5% and 10% for the cirrhotics and healthy controls, respectively. Diurnal heart rate variability and QTc disp were positively and negatively correlated in the cirrhotic patients and the control group, respectively. The correlation was insignificant in both groups (Figure 4).
4.5. Blood Pressure Variations
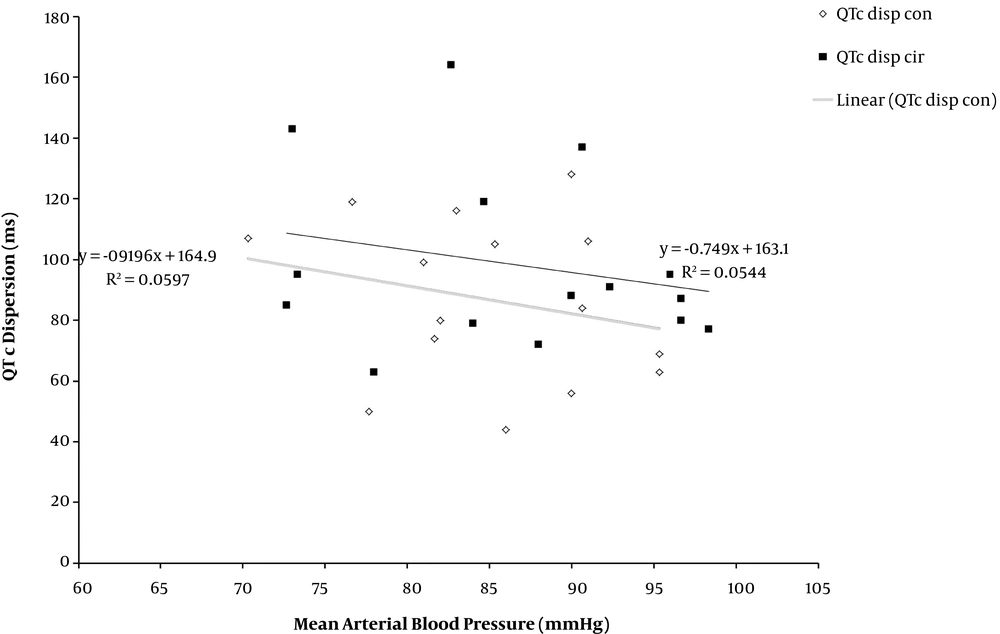
As shown in Figure 5, there was a negative linear correlation between QTc disp and the mean blood pressure over 24 hours in both groups (P = 0.78).
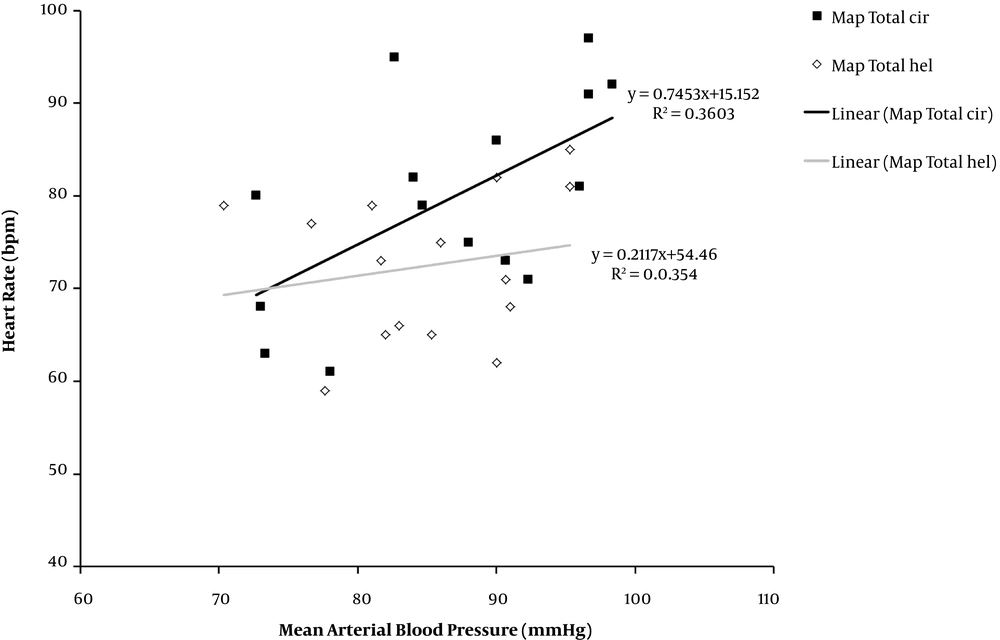
Blood pressure and heart rate were positively correlated in both groups, but they were greater in the cirrhotic group than healthy subjects (36% versus 3%) (Figure 6).
5. Discussion
In the present study, the diurnal variability pattern of QT, QTc, and QT dispersion was evaluated in a group of cirrhotic and a healthy control group. Data showed that QT interval and other ECG parameters were prolonged in cirrhotics compared to the healthy subjects, and QT prolongation was related to the severity of cirrhosis (Child-Turcotte-Pugh classification) as well. Longer QT interval was seen with higher Child-Turcotte-Pugh classification. These findings are in agreement with earlier studies (1, 4, 7, 8).
As previous studies have shown, autonomic dysfunction is common in cirrhotic patients that results in sympathetic overactivitym and elevated heart rate (9-11). To eliminate the effect of cycle length on QT interval, QT interval measurements were corrected using the Bazette’s formula (3, 4, 7, 8). QTc was significantly higher in cirrhotic patients than healthy subjects (P ≤ 0.05), and QT prolongation was proportional to the severity of the disease, as QTc interval.
In recent years, studies have been performed to define the exact reason of higher cardiac arrhythmia and sudden cardiac death (SCD) in cirrhotic patients (7, 9, 12, 13). Ion channel defects or cardiomyopathy (3, 7, 14, 15) and other diurnal haemodynamic variations have been mentioned as a potential cause (7).
A recent study by Tsiompanidis et al. (11) showed that cardiac autonomic neuropathy is common in cirrhosis and associated with the severity of the disease, but it has no significant relationship with the prolongation of QT interval in these patients. The diurnal pattern of the cardiac markers reported to be within the normal diurnal variation (7), unlike the pioneer study in this field (1997), which showed circadian variability pattern and increased QT, QTc, RR interval length, and heart rate variability (HRV) during the night. It was suggested that the relative parasympathetic tone be reflected. The parasympathetic withdrawal occurs during the awakening time. As a result, QT and heart rate decrease due to the autonomic disturbance and sympathetic effect. Interestingly, QTc prolongation occurs after waking up. They suggested that it’s likely to be related to the QT interval and rate of change in heart rate during adjustment to waking conditions. The decrease in RR interval occurs more rapidly than the QT interval, so, QTc interval length increases. Moreover, Molnar et al.’s study (16) showed that the diurnal QTc variation was not significant, but statistically valuable.
In this study, we found that circadian QT interval variation in healthy subjects had three peaks in the morning, afternoon, and night. QT interval length was longer during the nighttime than the daytime. The heart rate had been decreased during sleep until 4 - 5 AM. During waking hours, the QT interval showed a rapid decrease, and the heart rate increased. Circadian variation of QT interval was similar to cirrhotic patients and healthy subjects. QT interval length was shortened due to the declined heart rate during nighttime. The heart rate in patients with cirrhosis, unlike healthy subjects, showed an increasing trend in the early morning. This difference may represent the over-activity of the sympathetic nervous system or the vagal disturbance in cirrhotic patients, as suggested in the previous similar studies (9, 17, 18).
Diurnal QTc variability pattern is significantly different between cirrhotic patients and healthy subjects (11). In our study, the increased numbers of the peaks and the upward and rightward shift of curve were observed in cirrhotic patients. QTc values were longer during daytime than nighttime. Unlike healthy subjects, QTc values were constant in cirrhotic patients after 9-10 pm and slightly increased until 00-01 AM. It might be caused by a variable heart rate at these times.
Considering these findings, studies aimed at a more accurate evaluation of the diurnal hemodynamic variations are required in cirrhotic patients. In recent years, there was a limited investigation by Hansen et al. (7). They limited their investigation to circadian QTc variation and diurnal QTdisp patterns in cirrhotic patients, regardless of the dynamic diurnal variation of QTcdisp. They found a similar mean 24-hours QTc pattern and diurnal QTdisp variation in cirrhotic patients and the healthy subjects (7).
Previous studies investigated diurnal QTc disp variation pattern in cirrhotic patients along with other factors. They showed that QT and QTc could not predict ventricular arrhythmia, but QTdisp may predict it. QTdis ranges were between 30 to 60 ms in healthy subjects and 60 - 80 ms in patients who have coronary artery disease (CAD) (12, 19). We found one peak in diurnal QTdisp in the day and one at night in the healthy subjects, but just one peak at night in cirrhotic patients. Hansen also found a similar pattern for QTdisp (7).
Diurnal QTc disp variation pattern was determined using Bazett’s formula to eliminate the effect of heart rate. The pattern had two peaks in healthy subjects, and three peaks in the cirrhotic patients, whereas the third one was observed in the early morning. Moreover, the area under the curve was increased and sifted to the right. Our data on the diurnal pattern of QTc disp showed that cardiac arrhythmia incidence is higher in cirrhotic patients.
Present findings revealed no significant relationship between heart rate and blood pressure and QTdisp in both groups. Thus, more studies are required on other factors, although this was shown in other studies (7). Also, a 2-peak QTdisp diurnal pattern and a higher nighttime value of QTdisp has been reported in healthy subjects (19). Moreover, a correlation has been found between heart rate variability and QTdip raise the probability of sympathetic nervous system predominance in cirrhosis in addition to the effect of liver damage (11).
Various etiologies have been suggested regarding the prolonged QTc and normal QTdisp values, such as delayed repolarization in myocytes (7). Portal hypertension and variceal hemorrhage are shown to be associated with prolonged QTc (1, 17). An additional higher risk of gastroesophageal varices have been shown in cirrhotic patients during the night due to higher portal pressure (1, 13). In this study, it was impossible to assess this variable.
Ion channel abnormalities, especially the defects in potassium current and autonomic disturbances, could cause QTdisp in cirrhosis, associated with the increase in the systemic concentration of toxic substances (1, 8, 9).
5.1. Conclusions
In this study QTc was prolonged and increased with severity of cirrhosis, and its diurnal variation in cirrhosis was different from healthy subjects.