1. Background
Osteoarthritis (OA) can induce breakdown in the joint cartilage and underneath bone and lead to gradual degeneration of joint cartilage and its inefficiency. OA is more common in developed countries (1). Several mechanical, biological, and genetic factors are involved in this condition; some main factors are increasing age and obesity; therefore, it is common in the elderly (2). It is estimated that OA is the fourth cause of disability in the world, and the main concern is related to hip and knee joints, and considering its chronicity, the affected patients are forced to use long-term systemic and topical drugs. The prevalence of OA in the knee is approximately 10% and 13% in male and female elderly aged 60 and above, respectively (2). The massive pain caused by OA forced society to seek appropriate medical solutions (3). Since the OA difficulties are widespread and commonly happen at knee and hip, it can induce problems in walking and climbing stairs (4). Patients often feel pain while walking, running, and bending or kneeling. Arthritis also occurs after long movements; patients also experience stiffness of joints after a long time resting and waking up in the morning (4). OA may force the patients to undergo total hip and knee replacements (5), which puts the public health organizations and patients’ families in complex and troublous conditions (6).
Risk factors associated with OA and its distribution in the population can be described by means of epidemiological principles. OA can be defined by a scoring system using pathological, radiographical, and clinical approaches in five grades from 0 to 4 (7). In OA grade 2, the radiographic image shows a higher progression of osteophytes while the size of cartilage and the distance between the bones are normal; there is no friction between the bones, and synovial fluid is still adequate for joint movements. However, patients with grade 2 experience symptoms of the disease, such as pain after walking or running, stiffness in the joints after several hours of movement of the joints, and feeling discomfort when kneeling and bending. Grade 3 is considered as moderate OA; in this stage, the cartilage between the bones is damaged significantly, and the distance between the bones is narrow (8-11).
Many drugs are administrated to patients with OA that can cause relative improvement but have many side effects, which intensify in elderly patients.
Since the increase of age and obesity affect the incidence of OA in the society, and consequently, impose an economic burden and reduction in the quality of life; researchers may be interested to propose a better solution for the issue. Studies on OA in patients with joint symptoms may be considered by more clinical approaches than radiographic ones, because of the fact that most of the patients radiographically diagnosed with OA do not show any clinical symptoms (12).
According to the World Health Organization reports, many researches focus on natural remedies to relieve pain and compete with alternative medicines (13-15). In recent decades, the side effects of synthetic drugs made traditional medicine popular among patients. Herbal products, such as turpentine oil relieving the pain and inflammation of OA, are traditional medicines commonly used in Western and Central Iran (16, 17). Turpentine oil, used as an aseptic agent in ointments formulations, can be gained by distillation of pines or similar trees. Pistacia atlantica or Persian turpentine tree (also called baneh or wild pistachio tree) is the main source of turpentine in the region of Iran (18). P. atlantica and P. lentiscus are the major generic species and the source of oleoresin for economic and pharmaceutical production (19). Turpentine and rosin (the remained solid phase, after the distillation process) have various known applications, but the current study discussed only the application of turpentine in the formulation of a cream. Turpentine is used to treat gastric ulcer and digestive disorders. In recent studies, it is reported that P. atlantica has remarkable antioxidant, antibacterial, antifungal (20, 21), and anti-inflammatory activities. Turpentine oil is composed of terpene hydrocarbons, such as α-pinene, β-pinene, and limonene, as well as oxygenized terpenes, such as anethole, applied to the affected parts of human body skin (22, 23). It is reported that α-pinene inhibits a group of inflammatory agents (24). The resin part of oleoresin obtained from P. atlantica contains four- and five-ring triterpenes, such as masticadienonic acid, masticadienolic acid, morolic acid, oleanolic acid, and uronic acid, which can be detected in the acidic matters of resin fraction (23, 25, 26). However, depending on the source of the product, the proportions of these components may vary. The oleoresin obtained from P. atlantica is used to treat wounds, peptic ulcer, gastrointestinal disorders, and motion sickness (21).
2. Objectives
The present study aimed at evaluating the effect of the turpentine cream formulated in the current study on OA pain relief, and comparing the effects of oleoresin of P. atlantica tree with those of diclofenac gel on the improvement of the knee OA.
3. Methods
3.1. Plant Material
Pistacia atlantica was collected from May to July 2013 from the West of Iran, Kurdistan Province, and then authenticated by the Islamic Azad University, Pharmaceutical Sciences Branch (IAUPS) in Tehran, Iran, deposited as a voucher specimen (No: 267-HC).
3.2. Preparation of Essential Oil
In order to extract the essential oil, oleoresins (semi-solid extracts), the plants were subjected to hydro-distillation for four hours by means of a Clevenger apparatus (27). Then the essential oils dried in the anhydrous sodium sulfate solution (Sigma-Aldrich, Germany) were stored in amber vials at 4°C. The essential oil was analyzed with the capillary column BPX5 gas chromatography (Shimadzu GC-2010). The GC process was carried out using helium as a carrier gas and a flow rate of 1 mL/minute. The column temperature was initially 60°C, then raised to 270°C with the rate of 3°C/minute, and then to 270°C while the rate was changed to 15°C/minute.
3.3. Preparation of P. atlantica Cream
Initially, the oil phase mixture, including castor oil (as an adsorption enhancer), acetyl alcohol (as a stabilizer), stearic acid (as an oil phase constituent and stabilizer), and glycerol mono-stearate (as an emulsifier), was prepared. The formulated cream was prepared by mixing the oil phase and heating it at 70°C. Then the solution was mixed with the aqueous phase, and the temperature was raised to 75°C. The mixing was continued by agitation and adding triethanolamine, and the stirring continued until the cream was gradually cooled down. The preservative component was added at the end of the process.
3.4. Study Design
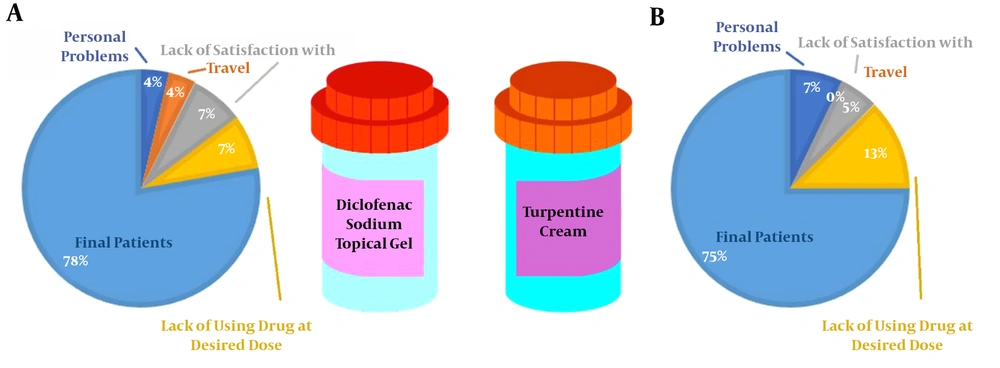
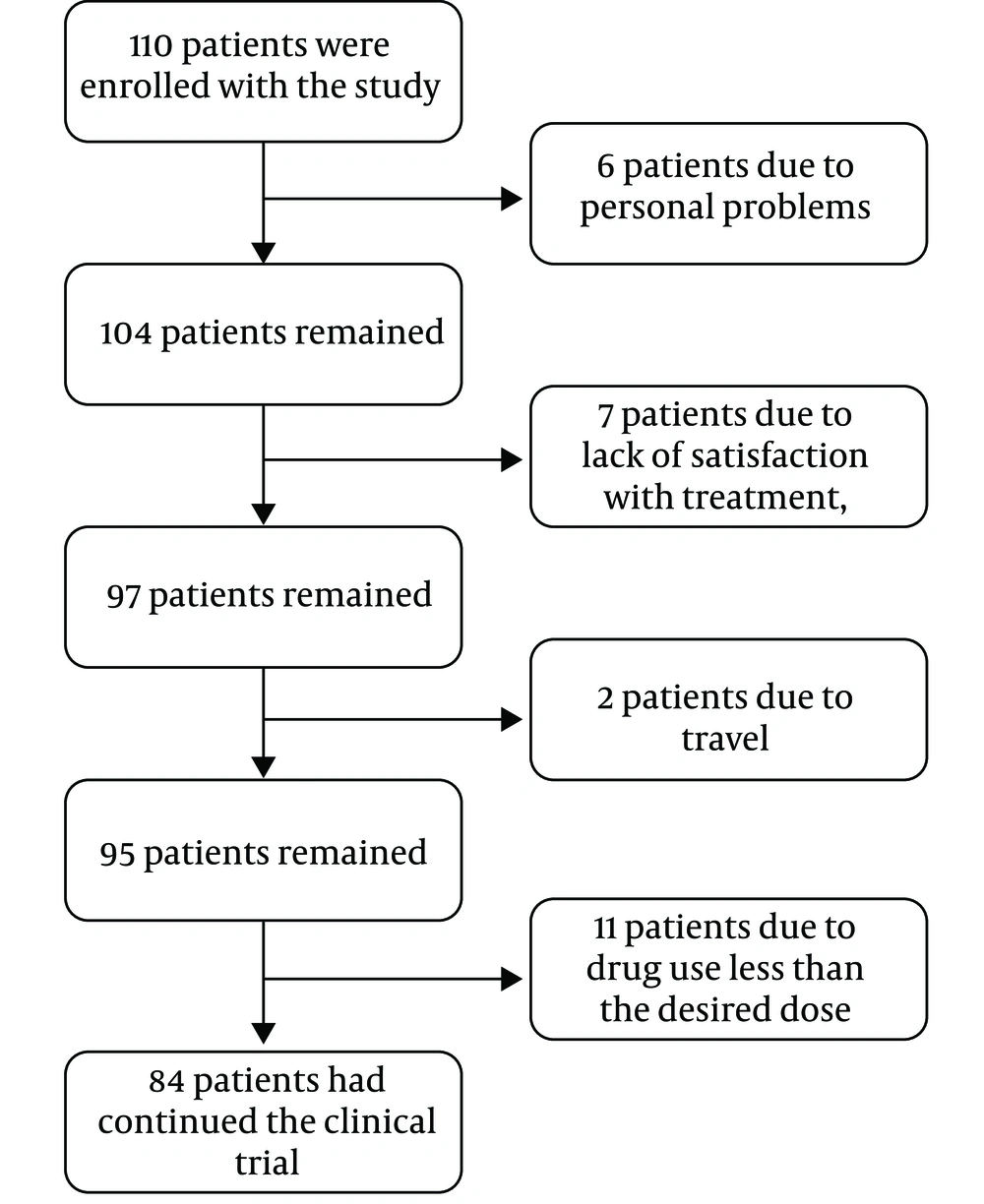
The current clinical trial was performed with parallel design, and diclofenac gel was used as the control drug. The study was conducted for 18 months at the Orthopedic Clinic of Buali Hospital, Iran. A total of 110 patients were initially enrolled in the study. After the exclusion of six patients due to personal problems, seven due to lack of satisfaction with the treatment, two due to travel, and 11 due to taking the drug less than the prescribed dosage and lack of proper cooperation, finally the study was completed with 84 patients (75 females and nine males) (Figures 1 and 2). The eligible patients diagnosed with OA grades 2 and 3 (mild to moderate level) by a specialist based on physical and radiographic examinations were selected. Before entering the study, the method and follow-up times were fully described to the patients, and they signed the consent form.
After obtaining the informed consent from the patients to participate in the trial, they were randomly assigned to two groups: (1) patients treated with P. atlantica cream and (2) patients treated with diclofenac gel; both the medicines were given three times daily in a dose of 2 g; in the P. atlantica cream group, 2 g of the cream was applied on the knee three times a day with massaging; then the subjects were asked to comply with the regimen for three months. They were also asked to refer to the clinic to receive the cream and follow-up the treatment process at certain times (2, 6, and 12 weeks after entering the treatment process), and contact with an announced phone number for any further questions. To evaluate the treatment process, the WOMAC questionnaire (The Western Ontario and McMaster Universities OA Index) was completed by the patients at the onset of the treatment and follow-up times. After 12 weeks, 84 patients completed the study, and then the effectiveness of the P. atlantica cream and diclofenac gel was compared by the patients’ satisfaction. Efficacy assessment: The WOMAC questionnaire, an instrument to measure symptoms and physical disabilities, was firstly developed for people with hip or knee arthroplasty (28, 29). The WOMAC measure evaluates the clinical changes in health status as a result of treatment interventions (30). WOMAC measures OA symptoms based on several indicators, including pain, stiffness of joints, and motor dysfunction. Motion functions that cover daily living activities include climbing the stairs, getting up from the chair, standing, bending, walking, riding and getting out of the car, shopping, putting on and taking off the socks, lying in bed, sitting, and doing light and heavy home management tasks (31). Many studies reported the reliability, validity, and responsiveness of the WOMAC questionnaire (30).
3.5. Ethical Considerations
The current study was designed and conducted in accordance with the principles of the Declaration of Helsinki, and was also approved by the Medical Ethics Review Board at the Islamic Azad University, Pharmaceutical Sciences Branch. All of the volunteers were asked to provide written consent to participate in the study, and the confidentiality and anonymity of the collected data were guaranteed. The study was registered with the Iranian Registry of Clinical Trials (No. IRCT201506073106N26). The study protocol was approved by the Ethics Committee of the Islamic Azad University, Pharmaceutical Sciences Branch (ethical code: 3332, dated: 20.06.2012).
3.6. Statistical Analysis
The current study data were expressed as mean ± standard error of the mean. All the gathered data were analyzed in Microsoft Excel 2016 and SPSS version 19 software using descriptive and analytical statistics of the chi-squared test and repeated measures ANOVA. P < 0.05 was considered as the level of significance.
4. Results
The participants were evaluated in terms of disease improvement by means of four defined stages in accordance with the WOMAC questionnaire as follows:
1. Symptoms: Knee inflammation, cracking sound (crepitation) by moving, knee lock, knee bending
2. Stiffness: Dryness of the joints after waking up in the morning and after resting intervals in a day
3. Pain: Frequency of pain experience and its severity in performing the activities, such as turning on the knee, knee bending, walking on the smooth surface, going up and down the stairs, night in bed, resting, bending to lift the objects, getting in and out of the car, shopping, putting on and taking off socks, getting out of bed, using toilette, and bathing
4. Daily function: Feeling pain while performing home management tasks, consisting of light home tasks (such as cooking) or heavy tasks (such as washing and moving heavy objects).
Patients’ demographic characteristics in the P. atlantica cream and diclofenac gel groups are presented in Table 1. In the current study, 37 females and five males were in the P. atlantica cream and 38 females and four males in the diclofenac gel groups. According to Table 2, there was no significant difference between the groups, and they were matched by height, weight, age, and body mass index (P < 0.05). Based on the obtained results, there was a direct relationship between the incidence of knee OA and overweight, as well as the incidence of knee OA and aging (P < 0.05). The first round of the WOMAC questionnaire completion, indicated a significant reduction in patients’ problems by both groups (P < 0.05). According to Table 3, there was a significant difference between the effects of P. atlantica cream and diclofenac gel in all the three follow-up times (2, 6, and 12 weeks) (P < 0.05).
| Index | P. atlantica Cream Group, Mean (SD) | Diclofenac Gel Group, Mean (SD) |
|---|---|---|
| Age (y) | 56.6 (10.5) | 56.2 (8.9) |
| Height (cm) | 162.8 (4.7) | 161.1 (4.0) |
| Weight (kg) | 69.5 (8.7) | 70.6 (8.4) |
| BMI (kg/m2) | 26.4 (3.1) | 27.2 (2.9) |
| Composition | Retention Time | Percentage | ||
|---|---|---|---|---|
| P. atlantica | P. atlantica Cream | P. atlantica | P. atlantica Cream | |
| α-pinene | 4.640 | 4.575 | 85.96 | 86.44 |
| Camphene | 4.935 | 4.875 | 1.29 | 1.36 |
| Verbena | 5.037 | 5.005 | 0.33 | 0.35 |
| β-pinene | 5.498 | 5.455 | 3.06 | 3.21 |
| β-myrcene | 5.728 | 5.690 | 0.15 | 0.20 |
| D-limonene | 6.070 | 6.025 | 0.34 | 0.41 |
| 3-carene | 6.194 | 6.155 | 0.39 | 0.39 |
| p-cymene | 6.509 | 6.465 | 0.52 | 0.51 |
| D-limonene | 6.603 | 6.565 | 1.45 | 1.43 |
| 1,8-cineole | 6.691 | 6.670 | 0.25 | 0.25 |
| α-terpinolene | 7.996 | 7.955 | 1.62 | 1.67 |
| Linalool | 8.241 | 8.210 | 0.11 | 0.15 |
| p-cymen-8-ol | 10.366 | 10.315 | 0.37 | 0.38 |
| α-terpineol | 10.500 | 10.450 | 0.91 | 0.90 |
| Myrtenol | 10.683 | 10.600 | 0.29 | 0.41 |
| Levoverbenone | 11.003 | 10.955 | 0.33 | 0.33 |
| bornyl acetate | 12.757 | 12.705 | 1.49 | 1.47 |
| Exo-2-hydroxycineol acetate | 14.051 | 14.010 | 0.14 | 0.14 |
| Studied Effect/Time | Symptoms of Knee Osteoarthritis | Knee Stiffness | Knee Pain | Knee Physical Activity | General Treatment of Knee Osteoarthritis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TC | DG | TC | DG | TC | DG | TC | DG | TC | DG | |
| Onset of treatment | 31 | 36 | 40 | 50 | 50.7 | 52.5 | 56.0 | 61.2 | 42.1 | 37.5 |
| 2nd week | 39.5 | 41.9 | 61.6 | 65.5 | 67.7 | 66.7 | 71.3 | 70.8 | 29.1 | 29.4 |
| Rate of variations | 8.5 | 5.0 | 21.5 | 15.5 | 17.0 | 13.6 | 15.3 | 9.6 | 13.1 | 8.1 |
| 6th week | 44.7 | 43.8 | 72.6 | 76.8 | 76.5 | 75.1 | 76.7 | 77.4 | 22.8 | 22.2 |
| Rate of variations | 13.7 | 7.7 | 32.5 | 26.8 | 25.8 | 22.6 | 20.7 | 16.2 | 19.3 | 15.3 |
| 12th week | 48.5 | 46.8 | 76.8 | 80.9 | 80.2 | 78.0 | 80.6 | 79.4 | 19.1 | 20.0 |
| Rate of variations | 17.1 | 10.7 | 91.5 | 30.9 | 29.5 | 25.5 | 24.6 | 18.2 | 23.1 | 17.5 |
| P value | 0.001a | 0.001a | 0.001a | 0.001a | 0.001a | 0.001a | 0.001a | 0.001a | 0.001a | 0.001a |
Abbreviations: TC, P. atlantica cream; DG, diclofenac gel
a Repeated measures ANOVA
According to the current study results, the patients’ problems in the 2nd and 12th weeks significantly reduced in both groups (P < 0.05) and significant differences were found between the effects of P. atlantica cream and diclofenac gel in all the three stages of the study (P < 0.05) (Table 4). P. atlantica cream and diclofenac gel significantly improved pain, symptoms, and problems of knee OA in the subjects. By comparing the improvement rate between the groups receiving P. atlantica cream and diclofenac gel, it was found that the P. atlantica cream could more significantly relieve pain and problems of patients with knee OA (P < 0.05) (Figure 1).
| Symptom of Knee Osteoarthritis | Knee Stiffness | Knee Pain | Knee Physical Activity | Overall Results Obtained by Treatments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TC | DG | TC | DG | TC | DG | TC | DG | TC | DG | |
| Onset of treatment | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| 2nd week | 27.2% | 16.2% | 53.9% | 30.9% | 33.6% | 27.1% | 27.3% | 15.7% | 31.0% | 21.6% |
| 6th week | 44.7% | 21.4% | 81.4% | 53.6% | 50.9% | 43.1% | 37.0% | 26.5% | 45.9% | 40.7% |
| 12th week | 56.2% | 29.7% | 92.0% | 61.9% | 58.2% | 48.5% | 43.9% | 29.8% | 54.7% | 46.6% |
Abbreviations: TC, P. atlantica cream; DG, diclofenac gel
5. Discussion
According to recent studies on the essential oil of P. atlantica using the distillation method and oleoresin essential oil using GC-mass apparatus, α-pinene accounts for about 70% to 98% of the components, which play a pivotal role in anti-inflammatory effects of P. atlantica oil (32-34). P. atlantica oil had the highest amount of α-pinene. Oleoresins of Pistacia species contain four- and five-ring triterpenes such as masticadienonic acid, masticadienolic acid, morolic acid, ursonic acid, and oleanolic acid. In a study conducted by Assimopoulou et al., P. lentiscus was used to determine the triterpenes of neutral and acid oleoresin fraction of Pistacia Spp. They indicated that they contain isomasticadienone acid, masticadienonic acid, and 28-norolean-17-en-3-one (35). The effect of triterpenes obtained from P. lentiscus and P. terebinhtus were compared by resent studies. According to their results, both species contained isomasticadienone acid, masticadienonic acid, and 28-nor lean-17-en-3-one (35, 36) in high levels of content. Jiner et al., realized that the substance extracted from P. terebinthus gall was effective in the treatment of chronic and acute inflammations. The results of their study showed that all the three triterpenes were effective in decreasing the inflammation caused by12-O-tetradecanoyl phorbol in mouse ear and edema created by phospholipase A2 in mouse leg. In addition, triterpenes inhibited the production of leukotriene B4 in mononuclear leukocytes induced by calcium ionophore (37). Based on the studies conducted by Asipur et al., oleoresins of Pistacia Spp. (full oleoresin of P. atlantica), have bioactive triterpenes with proven anti-inflammatory properties. The therapeutic effects of an ointment containing cinnamon, ginger, P. atlantica, and sesame oil plus the salicylate ointment was examined on patients with OA at two-, four-, and six-week intervals. The results showed that the application of the formulated ointment plus salicylate ointment clinically reduced the pain and stiffness of the joints in the morning and improved the limitation of movement at these joints (38). Minaeian et al., conducted a study to examine the anti-inflammatory properties of essential oils obtained from P. atlantica and P. atlantica in the treatment of colitis in Wistar rats. The effect of the oral form of P. atlantica essential oil was similar to prednisolone and hydrocortisone, and could inhibit all inflammatory symptoms of colitis. However, its rectal form did not show a specific anti-inflammatory effect. The results indicated that the P. atlantica cream had analgesic activities. In another study conducted by Sosa et al., the tail-flick test was used to evaluate the analgesic effect of α-pinene. The obtained results indicated that its analgesic effect was similar to that of morphine for 30 and 90 minutes; however, the analgesic effect of α-pinene lasted 150 minutes, showing that its effect lasts longer than that of morphine. A review of these studies can scientifically confirm the effectiveness of P. atlantica ointment in the improvement of the joint pain and problems.
In the current study, the symptoms, including knee OA, knee joint cracking sound (crepitation), knee lock, and inability to open and close the knee, respectively in the P. atlantica and diclofenac groups were 27.2% and 16.2% after two weeks, 44.1% and 21.4% after six weeks, and 52.6% and 29.7% after 12 weeks.
In addition, improvement in the symptoms of joint stiffness in the morning and after resting during the day was reported 53.9% and 30.9% respectively in the P. atlantica and diclofenac groups after two weeks, 81.4% and 53.6% after six weeks, and 83% and 61.9 after 12 weeks (Table 4). Symptoms of pain (including relief level, recurrence time, and pain during turning on the knee, during opening and closing of the knee, walking on the smooth surface, going up and down of the stairs, at night in bed, and at the time of sitting, lying, and standing) were examined in the P. atlantica and diclofenac groups, and respectively, an improvement of 33.6% and 27.1% was reported after two weeks, 5.9% and 43.1% after six weeks, and 58.2% and 48.5% after 12 weeks. Daily living activities, including the difficulty in performing the tasks such as sitting and getting up, bending to lift the objects, walking, getting on and out of the car, shopping, putting on and taking off the socks, walking, while sleeping in bed, bathing, and performing light and heavy home management tasks were evaluated. According to the results, an improvement of 27.3% and 15.7% after two weeks, 37% and 26.5% after six weeks, and 43.9% and 29.7% after 12 weeks were observed in the P. atlantica and diclofenac groups, respectively.
Results indicated that the short-term use of P. atlantica cream satisfied patients, and the continuation of its application increased the effects. The current study results indicated a significant difference between the P. atlantica cream and diclofenac gel in terms of effectiveness, and a significant difference in the symptoms of patients with OA between the two drugs before and after treatment. In other words, P. atlantica cream significantly acted better than diclofenac in all studied domains. Since Iran is one of the main suppliers of P. atlantica worldwide, domestic herbal products with low prices and side effects can help both the economy of the community and patients.
5.1. Conclusion
P. atlantica cream containing oleoresin, alone and in combination with other systemic drugs, reduced pain, inflammation, and restrictions of joint movement in patients with mild to moderate (grades 2 and 3) knee OA. The anti-inflammatory activity of P. atlantica cream is mainly caused by the analgesic effect of α-pinene that inhibits some enzymes involved in inflammation caused by OA. It does not have the side effects of conventional arthritis treatments in reducing the pain and inflammation. Based on the studies, the most common problem of OA is the joint stiffness that reduces the ability of patients to perform daily living activities. It can be reduced by the topical application of P. atlantica cream that can be used as an alternative to other drugs such as diclofenac that has complicated side effects.