1. Background
As one of the most common gynecological disorders, endometriosis is an estrogen-dependent disease characterized by the presence of endometrial glands and stroma outside the uterine cavity and musculature (1-5). Endometriosis affects 5 to 10% of women of reproductive age (4). The most common sites of endometriosis are the ovarian surface and the pelvic peritoneum, which may lead to pelvic inflammation, adhesions, chronic pain, and infertility (1-6). While the exact etiology of endometriosis is still unknown, some theories suggested that retrograde menstruation, coelomic metaplasia, embryonic cell rests, lymphatic and vascular dissemination are associated with increased risk of endometriosis (1-5).
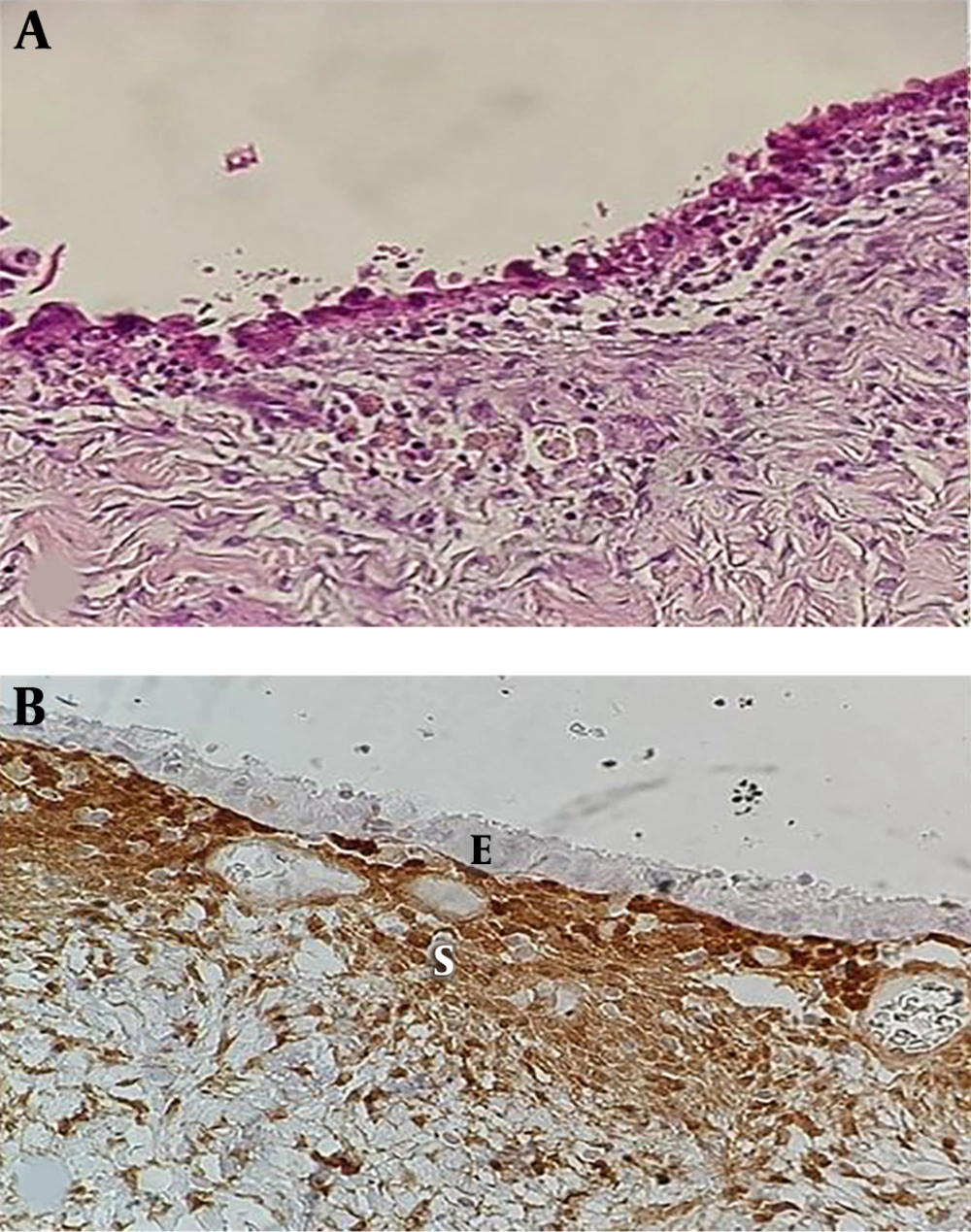
The epithelial lining of ovarian endometriosis is similar to its normal uterine counterpart that can undergo metaplastic, hyperplastic, atypical changes, and even malignant transformation (7).
Atypical endometriosis is defined as epithelial lining of endometriosis with large hyperchromatic or pale pleomorphic nuclei, increased nucleocytoplasmic ratio, and cellular crowding, stratification, or tufting (3). Several studies have reported that atypical endometriosis possesses a precancerous potential to a transit benign endometriosis to carcinoma (2, 4, 8, 9). Clear cell carcinoma and endometrioid adenocarcinoma are two predominant subtypes of endometriosis associated with ovarian cancer (EAOC) (2, 10).
PTEN (phosphatase and tensin homolog deleted on chromosome 10) is one of the mutated human tumor-suppressor genes. The PTEN gene has both lipid and protein phosphate activity and a central negative regulator of the PI3K/AKT signaling cascade. Loss of PTEN gene can cause aberrant cell growth, proliferation as well as an escape from apoptosis, in addition to abnormal cell dispersion (11, 12). Many neoplasias are stimulated by loss of PTEN protein, including prostate, breast, and glial tumors. Moreover, it frequently occurs in both endometrial and ovarian carcinomas. A decrease in PTEN expression tends to be associated with malignant features of the endometrium with a significant difference in PTEN immunoreactivity between groups of normal endometrium, hyperplastic changes and carcinoma (13). Also, LOH at 10q23.3 (PTEN protein loss) has been reported in endometrial cysts of the ovary, ovarian endometrioid carcinomas, and clear cell carcinomas (5, 14-16).
Ki-67 is one of the most widely used nuclear markers, which expresses during all active cell cycle phases (17); hence, in the present study, its expression was examined to assess the proliferative activity.
2. Objectives
This study aimed to determine the immunohistochemistry expression of ki67 and PTEN markers in typical endometriosis, atypical endometriosis, and EAOC.
3. Methods
3.1. Study Design
This retrospective study was conducted on all patients diagnosed with ovarian endometriosis undergoing surgery in two major gynecology centers affiliated to Shiraz University of Medical Sciences (SUMS) (Shiraz, Iran) between 2012 to 2016. In total, 41 cases with typical endometriosis and 25 cases with atypical endometriosis were studies. The study protocol was approved by the Institutional Review Board (IRB) and the Ethics Committee of SUMS (code: IR.sums.med.rec.1395.s179). All ethical considerations were observed at various stages of the study.
Atypical endometriosis was diagnosed based on the histopathological criteria suggested by LaGrenada and Silverberg (8). These features included large pleomorphic hyperchromatic or pale nuclei, eosinophilic cytoplasm, tufting, crowding, and stratification. Patients were diagnosed with atypical endometriosis if at least three of these criteria were abnormal.
The inclusion criteria were adequate epithelial cells and endometrial stroma for typical endometriosis and adequate atypical changes in endometriosis for atypical endometriosis. The exclusion criteria for selecting atypical endometriosis were having acute inflammatory cells in the epithelial cells (inflammation), ciliated metaplasia, and severe hemosiderosis.
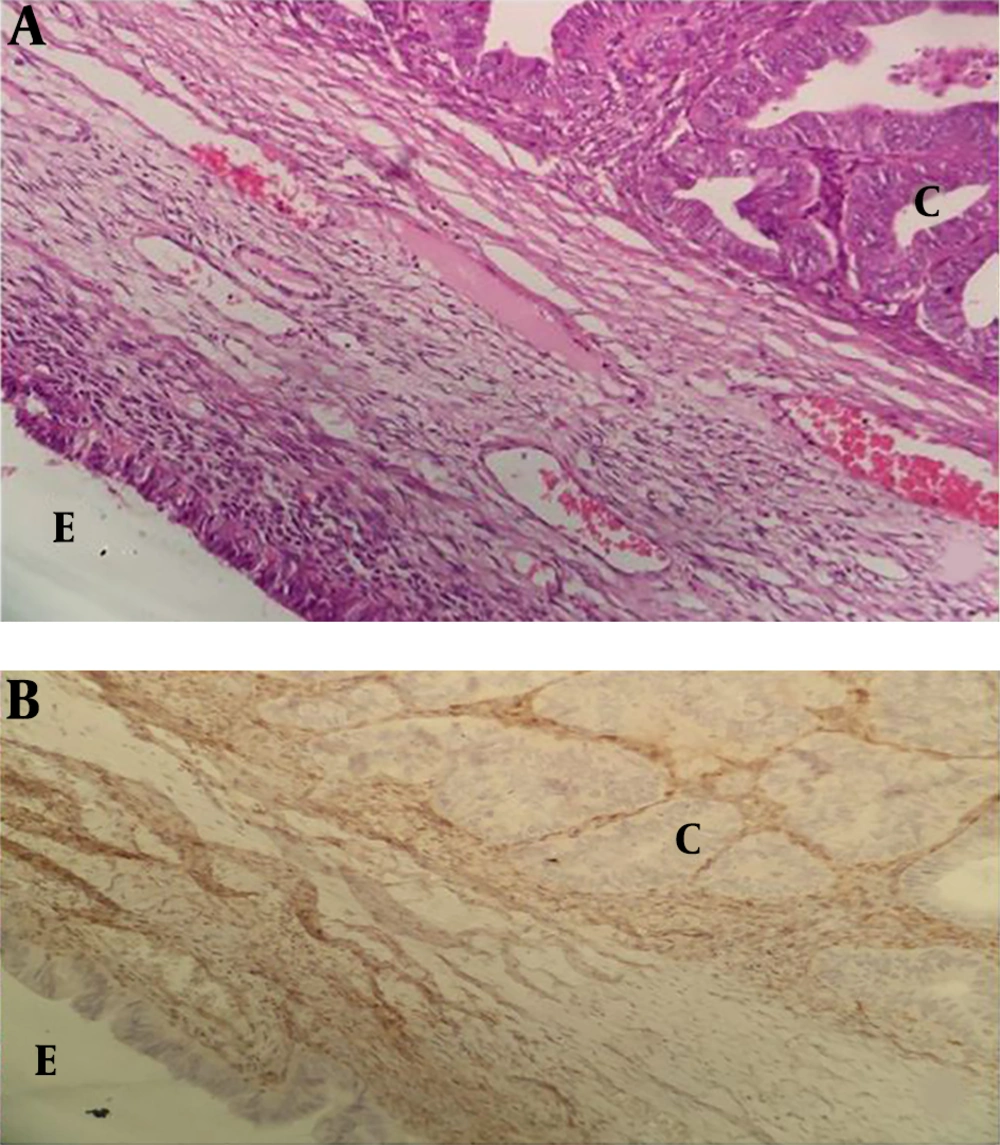
For the third group, 17 ovarian cancer cases with endometriosis who had enough tissue for immunohistochemistry were selected from a previously published study (18). The other seven cases were new ones selected from the pathology archive. The criteria of endometriosis-associated with ovarian cancer were as follows: (1) The presence of both a malignant tumor and endometriosis in the same ovary; (2) The demonstration of ovarian cancer only from endometriosis; (3) The presence of endometrial stroma surrounding endometrial glands, and (4) Indications of a transition from benign endometriosis to the malignant epithelium (19).
3.2. Immunohistochemistry Staining
Immunohistochemical staining (IHC) for Ki67 and PTEN was performed manually. Paraffin-embedded, formalin-fixed blocks were sectioned with 4-micron thickness. Two unstained tissue sections were collected for IHC staining with Ki67 (monoclonal Rabbit antihuman antibody, clone SP6-BIOCARE) and PTEN (monoclonal mouse antihuman antibody, clone 6H2.1-product code: M3627, DAKO).
The sections were deparaffinized in xylene (three times, each for 10 minutes) and gradually rehydrated with ethanol (100%, 96%, and 70%, respectively) and distilled water (two minutes). Then, it was rinsed by phosphate buffer saline (P.B.S) with PH: 6.0 for 5 minutes. After blocking the endogenous peroxidase activity by treating hydrogen peroxidase with P.B.S (H2O2 0.3% for 20 minutes), until no bubble was observed on the surface of the slide, sections were incubated at 95°C to 98°C for 40 minutes in TE buffer (PH = 9) as an antigen retrieval step. After drying the slides and using dakopen, the serum of gout, which was diluted by P.B.S to 10%, was applied for 20 minutes. Afterward, we applied the primary antibody of PTEN (with a dilution of 1/200) and Ki67 to each section of the cases and incubated them overnight at 4°C. After 24 hours, the sections were washed using P.B.S. (two times, each for 10 minutes).
For the secondary antibody, the envision peroxidase system was used on dried slides (biotynilated link anti-mouse and anti-rabbit), which were then washed with P.B.S (2 × 10 times).
The product was revealed by incubation with diaminobenzidine (DAB’S 3000, DAKO) for 10 minutes, followed by a washing phase with P.B.S for 5 minutes. The nuclei were counter-stained with hematoxylin. Finally, dehydration and mounting were performed.
The cytoplasm and nuclei of endometrial stromal cells were used as the positive internal control for PTEN.
All IHC staining slides were assessed under a light microscope. Cytoplasmic and nuclear staining for PTEN was categorized as: Negative (-) if less than 10%, positive (+1) if between 10-50%, and positive (+2) if greater than 50% of epithelial cells were stained. The intensity of positive cases was grouped as: mild (+1), moderate (+2), and strong (+3). Nuclear staining for Ki67 was determined by the percentage of the total cells that were stained in epithelial and stromal cells, separately.
3.3. Statistical Analysis
Data were analyzed using SPSS version 16 for windows (SPSS Inc., Chicago, IL, USA). The data were presented as mean ± standard deviation (SD). Statistical significance was considered when P value < 0.05. The ANOVA and chi-square tests were used to compare quantitative and qualitative variables between the groups, respectively. In case the number of cells, which have expected frequencies less than 5, exceeded 25% of all cells, the Fisher exact test was used to perform intra-group qualitative comparisons.
4. Results
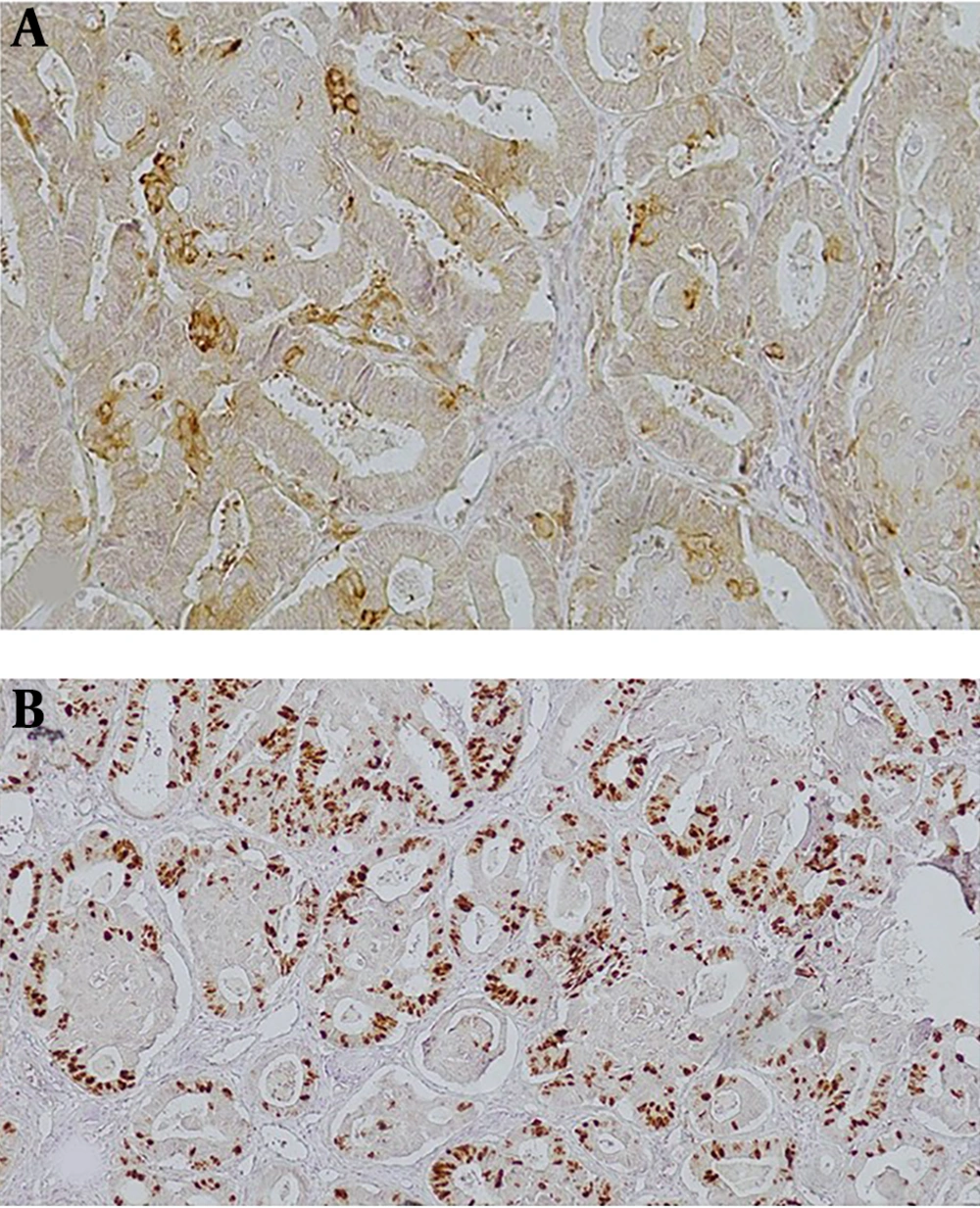
The youngest and oldest patients with typical endometriosis, atypical endometriosis, and EAOC were 19 and 51 years (30.5 ± 7.13), 21 and 46 years (30.8 ± 7.48), and 20 and 76 years (45 ± 11.7), respectively (P < 0.001). In total, 260 and 25 cases of endometriosis and atypical changes were detected, respectively. The prevalence of atypical endometriosis was about 9% (25 out of 260). In patients with EAOC, 10 out of 24 (41.7%) had serous carcinoma, 8 out of 24 cases (33.3%) had endometrioid carcinoma, and 6 out of 24 cases (25%) had clear cell carcinoma. PTEN expression in epithelial cells was observed in all typical endometriosis cases. Strong immunoreactivity (3+) was observed only in typical endometriosis. PTEN loss was observed in 8% (2 out of 25) of patients with atypical endometriosis, and 50% (12 out of 24) of patients with EAOC (P < 0.001) (Figures 1 and 2). Of 12 patients with EAOC, 6 (50%) were endometrioid carcinoma, 4 (33.3%) serous carcinoma, and 2 (16.6%) clear cell carcinoma. For all 12 PTEN loss cases, the PTEN loss pattern in endometriosis adjacent to ovarian cancer was similar to that of ovarian cancer. PTEN expression, the intensity in epithelial cells, and stromal cells are shown in Tables 1 and 2.
| Groups | Total | Epithelial Cells | Stromal Cells | |||||
|---|---|---|---|---|---|---|---|---|
| < 10% | 10% - 50% | > 50% | P Value | 10% - 50% | > 50% | P Value | ||
| Typical endometriosisA | 41 | 0 (0) | 6 (14.5) | 35 (85.4) | PAB = 0.001 | 5 (12.2) | 36 (87.8) | PAB = 1 |
| Atypical endometriosisB | 25 | 2 (8) | 8 (32) | 15 (60) | PBC = 0.005 | 3 (12) | 22 (88) | PBC = 0.025 |
| EAOCC | 24 | 12 (50) | 4 (16.7) | 8 (33.3) | PAC = 0.0001 | 10 (41.7) | 14 (58.3) | PAC = 0.013 |
| Groups | Total | Epithelial cells | Stromal Cells | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1+ | 2+ | 3+ | P Value | 1+ | 2+ | 3+ | P Value | ||
| Typical endometriosisA | 41 | 17 (41.5) | 18 (43.9) | 6 (14.6) | PAB = 0.074 | 3 (7.3) | 15 (36.6) | 23 (56.1) | PAB = 0.926 |
| Atypical endometriosisB | 25 | 16 (64) | 9 (36) | 0 (0) | PBC = 0.037 | 2 (8) | 10 (40) | 13 (52) | PBC = 0.030 |
| EAOCC | 24 | 22 (91.7) | 2 (8.3) | 0 (0) | PAC = 0.0001 | 5 (20.8) | 15 (62.5) | 4 (16.7) | PAC = 0.004 |
PTEN stains the stroma as well as the epithelial component with different intensity levels. Stromal PTEN staining was detected in all specimens and was considered as the positive internal control. In typical endometriosis and atypical endometriosis, more than 50% of the cases had PTEN stromal staining intensity of 3+, and in groups of EAOC, more than 50% of the cases had PTEN stromal staining intensity of 2+ (P = 0.14).
Ki67 immunoreactivity in epithelial and stromal cells is shown in Table 3. In total, 7.3% of typical endometriosis, 8% atypical endometriosis, and 33.3% of EAOC had Ki67 staining in more than 50% of the epithelial component (Figure 3). Typical and atypical endometriosis were similar in Ki67 staining (in more than 50% of the epithelial component) (P = 0.7), and both of them were different from EAOC (P < 0.05).
| Groups | Total | Ki67 Staining in Epithelial Cells | Ki67 Staining in Stromal Cells | |||||
|---|---|---|---|---|---|---|---|---|
| 10% - 30% | 31% - 50% | > 50% | P Value | 10% - 30% | > 30% | P Value | ||
| Typical endometriosisA | 41 | 26 (63.4) | 12 (29.3) | 3 (7.3) | PAB = 0.778 | 30 (73.2) | 11 (26.8) | PAB = 0.025 |
| Atypical endometriosisB | 25 | 18 (72) | 5 (20) | 2 (8) | PBC = 0.0195 | 23 (92) | 2 (8) | PBC = 0.607 |
| EAOCC | 24 | 8 (33.3) | 8 (33.3) | 8 (33.3) | PAC = 0.015 | 21 (87.5) | 3 (12.5) | PAC = 0.355 |
5. Discussion
In the present study, the prevalence of atypical endometriosis was about 9% (25 out of 260), which is consistent with the results of a meta-analysis that reported a prevalence of 8% (19). In this meta-analysis, five studies that in total, comprised 1109 patients with endometriotic lesions are reviewed, and a total of 89 cases of severe atypical endometriosis are identified (19). In the present study, the mean age of typical and atypical endometriosis cases was about 30 years, in which patients in the EAOC group were significantly different concerning the variable of age (the mean age was 45 years). Another study conducted on a sample of patients with endometriosis in Iran with a mean age of 27.9 ± 6.1 years has reported similar results (20). The higher mean age of patients in the EAOC group, compared to those in the endometriosis and atypical endometriosis groups, can be attributed to the long-lasting process of progression from endometriosis to atypical endometriosis and to carcinoma. Studies conducted by Mangili et al. (21) and Akbarzadeh et al. (18) reported a mean age of 55 ± 10 and 49.93 ± 9.36 years for patients with endometrioid ovarian cancer-associated endometriosis, respectively, which was lower than the mean age of patients with only endometrioid ovarian cancer (i.e., 62 ± 12 years) (21). These findings are also consistent with those of the present study. Patient age, when diagnosing ovarian endometriosis, postmenopausal status, is a risk factor for the development of EAOC (22).
Several studies have suggested that atypical endometriosis is a transition between benign endometriosis and EAOC (2, 4, 7, 8). In 1925, Sampson was the first to describe the malignant transformation of endometriosis to ovarian carcinoma (23). Moll et al. (24) reported a chronological association between atypical endometriosis and ovarian carcinoma in a woman with clear cell carcinoma three years after ovarian cystectomy of an endometrioma with severe atypical changes.
Okamura and Katabuchi (25, 26) presented evidence of a direct transition from the endometriotic gland to atypia to carcinoma in endometrioid carcinoma arising from an ovarian endometriotic cyst. In a study by Akbarzadeh et al. (18), 25.4% (28 out of 110) of ovarian cancer cases had endometriosis. Twenty-three cases had typical endometriosis, 14 cases atypical endometriosis, and 19 cases, both typical and atypical endometriosis. In 11 cases, a transition from atypical endometriosis to carcinoma was observed (18).
In the present study, 50% of the EAOC cases showed PTEN loss in epithelial cells of endometriosis. A significant difference was found concerning the PTEN loss between EAOC cases compared to both typical and atypical endometriosis. Seventy-five percent (6 of 8) of endometrioid carcinoma, 40% (4 of 10) of serous carcinoma, and 33.3% (2 of 6) of clear cell carcinoma had PTEN loss in both epithelial cells of carcinoma and adjacent endometriosis. Moreover, endometrioid carcinoma was found as the most common carcinoma, a result found in a study by Djordjevic et al. (27) as well.
In all 12 PTEN loss cases, the pattern of PTEN loss in endometriosis adjacent to ovarian cancer was similar to ovarian cancer, which is in line with the study by Sato et al. (16). They identified synchronous endometriosis in five cases of endometrioid and seven cases of clear cell carcinoma. Three out of five cases of endometriosis carcinoma with endometriosis had LOH of PTEN common to both the carcinoma and the endometriosis. Three out of seven cases of clear cell carcinoma with endometriosis had LOH of PTEN common to both the carcinoma and the endometriosis (16). These findings revealed that the PTEN loss pattern is similar in ovarian cancer and atypical endometriosis adjacent to it, indicating that PTEN loss could be an early event in the tumor development pathway from endometriosis to ovarian cancer. Obata et al. (15) detected LoH at 10q23.3 in 43% of the endometrioid carcinomas, and 28% of the serous carcinomas; however, they reported that LoH seemed to be infrequent in other histological subtypes of epithelial ovarian cancers. Djordjevic et al. (27) evaluated PTEN loss in ovarian cancer and found PTEN loss in 64% (98 of 154) of all ovarian cancer. Out of 98 cases with PTEN loss, 75 (out of 100; 75%) were endometrioid carcinoma and 2 (out of 4; 50%) were clear cell carcinoma. In total, 40% (21 of 52) of other types of ovarian carcinoma with no PTEN loss were in serous carcinoma. With gene sequencing, PTEN sequencing abnormality was detected in 43% (66 out of 154) of all ovarian cancer and 51% (51 out of 100) of endometrioid carcinoma (27).
Ballouk et al. (28) reported that half of the atypical endometriotic cysts showed DNA aneuploidy, suggesting that these atypical endometriotic cysts have the potential to change to invasive epithelial malignancies. Martini et al. detected PTEN loss protein expression in 15% of endometriosis cases (14). This finding suggests that inactivation of the PTEN is an early event in the malignant transformation of endometriosis and atypical endometriosis could be a precursor of ovarian carcinoma.
Ballouk et al. (28) and Martini et al. (14) also mentioned PTEN loss in atypical endometriotic cysts (13). In the present study, 8% (2 of 25) of atypical endometriosis showed PTEN protein loss. However, there was no significant difference between typical endometriosis and atypical endometriosis. The following reasons can be considered as the possible explanations for this event: (A) transformation of any precursor cancerous lesion to carcinoma in the body organs is a long-standing process. As mentioned earlier, the mean age of patients with EAOC was significantly higher than patients with atypical and typical endometriosis, which suggests this point; (B) In this study all atypical changes in endomeriotic cysts of ovary were focal. Extensive sampling of endometriotic cysts is necessary to find more foci of atypical change; (C) The sample size of the present study was limited. Hence, studies with larger sample sizes are recommended to reveal the role of PTEN loss in the pathogenesis of atypical endometriosis. High-intensity expression of PTEN was only detected in typical endometriosis. None of the EAOC cases showed PTEN staining with 3+ intensity, and 83.3% (10 out of 12) of the cases with PTEN showed 1+ intensity. The results of a similar study by Sarmadi et al. (13) revealed that the intensity of PTEN reaction was significantly higher in the group with proliferative endometrium than patients with hyperplastic endometrium and endometrioid endometrial carcinoma.
Several IHC studies reported an association between the ki67 index and biological behavior. Hence, it seems that the ki67 index can provide valuable prognostic information in some cancers (7). Ogawa et al. (7) detected a significant difference between the Ki-67 indices of typical endometriosis, atypical endometriosis, and ovarian cancer (2.7 ± 0.90, 9.9 ± 1.73, and 23.1 ± 3.29, respectively). Atypical endometriosis revealed a proliferative activity intermediate to those of typical endometriosis and EACO, which suggests its role as a precancerous lesion (7). In the present study, 7.3% of typical endometriosis, 8% of atypical endometriosis, and 33.3% of EAOC had Ki67 staining in more than 50% of the epithelial cells.
Typical and atypical endometriosis had similarities in Ki 67 staining (P = 0.7), and both of them were different from EAOC. Several studies showed that ki67 indices in premalignant lesions and carcinoma in situ of the breast (29) and biliary tract (30) are lower than those associated with invasive carcinoma. Therefore, based on the results, premalignant lesions had a lower ki67 index than carcinoma.
In summary, the pattern of PTEN loss in endometriosis adjacent to ovarian cancer was similar to ovarian cancer. This result indicates PTEN loss could be an early event in the tumor development pathway from endometriosis to ovarian cancer, and atypical endometriosis can be considered as a precursor of ovarian carcinoma. Overall, these findings suggest that ovarian endometriosis is not as harmless as it seems because of its association with atypical endometriosis adjacent to ovarian cancer. Therefore, the follow-up of patients with atypical endometriosis is necessary.