1. Background
Most athletes use exercise supplements comprising anabolic-androgenic steroids (AAS), such as testosterone, to improve performance (1). Testosterone was first isolated in 1935, and then its synthesis methods were founded by Butenandt and Ruzicka (2). In the early 1950s, AASs were initially introduced to muscle building, and their use gradually moved on to other sports to increase muscle strength. Ultimately, the Olympic games and other athletic associations banned the use of these supplements in 1967 (3). Testosterone and its derivatives increase fat-free mass, lean muscle mass, overall body weight, skeletal muscle strength, and athletic performance (4-6). There are several side effects associated with the use of androgens, including testicular atrophy, gynecomastia, dyslipidemia, infertility, and severe liver damage (4, 7). There are three distinct patterns of liver injury due to AAS, including peliosis hepatis, liver tumors, and rarely nodular regenerative hyperplasia (NRH), which can occur with any route of administration. The use of steroid supplements is becoming a primary public health concern, with evidence of increased use among men over 40 and young gym attendees (8).
Liver failure is closely related to the incidence of mitochondria dysfunction. Mitochondria are the most critical organelles for energy metabolism (9, 10). Mitochondria perform their biological function through fusion and continuous divisions (11) using many proteins, including dynamin-related protein (DRP1), mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and mitochondrial fission one protein (Fis1) (12-14). Mitochondrial shape maintenance is dependent on the balance between fission and fusion events. Mitofusins (Mfn2 and Mfn1) were revealed to promote endomembrane docking and outer membrane fusion (15). DRP1 and Fis1 were displayed to induce mitochondrial fission and self-assemble into spiral- and ring-like structures that could fit the size of mitochondrial constriction sites (16, 17). Based on studies, Mfn2 has an essential effect on cell apoptosis and autophagy and plays distinct roles among different tissues (11, 18).
Date palm pollen (DPP or Phoenix dactylifera L., from the Palmae family) is the male reproductive cells of palm flowers, which was used by the early Chinese and Egyptian people as a rejuvenating therapeutic agent (19). DPP extracts were shown to possess a myriad of pharmacological properties, like antioxidants (20). Furthermore, it has been used in traditional medicine to treat various disorders, including liver and kidney diseases, memory impairment, complete paralysis, inflammation, fever, consciousness loss, and many neurological diseases (21, 22).
It has been shown that strength and hypertrophy are developed by resistance training (RT) for people with wasting diseases and elite athletes seeking to maximize their performance (23). Testosterone can also increase strength and hypertrophy, even without resistance training in both young (24) and older men (25). Many studies have demonstrated the role of RT in increasing blood serum testosterone (26, 27).
2. Objectives
In the present study, we tested the effect of DPP Extract (DPPE) and testosterone alone (T) and in combination with resistance exercise on liver tissue in adult male rats. To this end, the gene expression of MFN1 and protein expression of DRP1 and MFN1 were evaluated. Also, cell apoptotic status in the liver was assessed using tunnel assay. Moreover, collagen deposition in the liver was detected by Masson’s trichrome staining.
3. Methods
3.1. Animal and Ethic
Thirty 8-week Sprague–Dawley male rats were purchased from the Tehran Pasteur Institute. All animals were housed in standard cages with standard laboratory conditions (temperature was 22°C, the humidity was about 45%, and light-dark cycles were 12/12 hours) and free access to food and water. To perform the project, the animals were randomly divided into six groups (n = 5 in each group), including (1) Control (C) group, (2) RT-treated group, (3) DPPE-treated group, (4) Testosterone (T)-treated group, (5) DPPE+RT- treated group, and (6) T+RT-treated group.
3.2. Animal Treatments
We gave 100 mg/kg DPPE to animals of groups 3 and 5 (five days/week for four weeks) (28). Then, 100 mg testosterone enanthate was purchased from the pharmacy and subcutaneously injected into animal groups 4 and 6 at a dose of 2 mg/kg daily (five days/week for four weeks) (29).
3.3. RT Program
Climbing from the vertical ladder (110 cm height, 80-degree incline, and 2 cm separation between steps) were used for the exercise of animal groups 2, 5, and 6. After being familiarized with the ladder without load, the first load was 75% of each rat’s body weight, and the load was increased by 30 g for each following set until the rats were no longer able to travel the entire ladder. The second phase of progressive training was begun after 48 hours, consisting of four to seven ladder climbs a day for four weeks. Each of the first four sets of climbing was performed using loads that were 50, 75, 90, and 100% of the rat’s body weight. After that, the weight became heavy until the rats could no longer climb the ladder (30).
3.4. DPPE Preparation and Biochemical Methods
Phoenix dactylifera were collected from palm groves (Shahdad, Kerman, Iran). Extracted, and the efficiency of extraction was 87.17%. The extraction process, tissue histological assay, Masson’s trichrome staining, immunohistochemistry, and real-time PCR were carried out exactly according to the article by Nazarian et al. (31). Table 1 summarizes primers used for the real-time PCR.
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| MFN1 | CTCCTG TAATCTTGCCTG | ATCGGATCTTTTTTGTTTCAGC |
| GAPDH | AAGTTCAACGGCACAGTCAAGG | CATACTCAGCACCAGCATCACC |
3.5. Statistical Method
Data were reported based on the mean ± standard deviation (SD). A two-way analysis of variance (ANOVA) was used to determine the effect of RT, DDPE, and T and their interaction with the present study’s variables. The Bonferroni post hoc test was used to determine the location of the difference. The significance level was also considered for all calculations (P < 0.05). All statistical analyses were done using SPSS software version 24.
4. Results
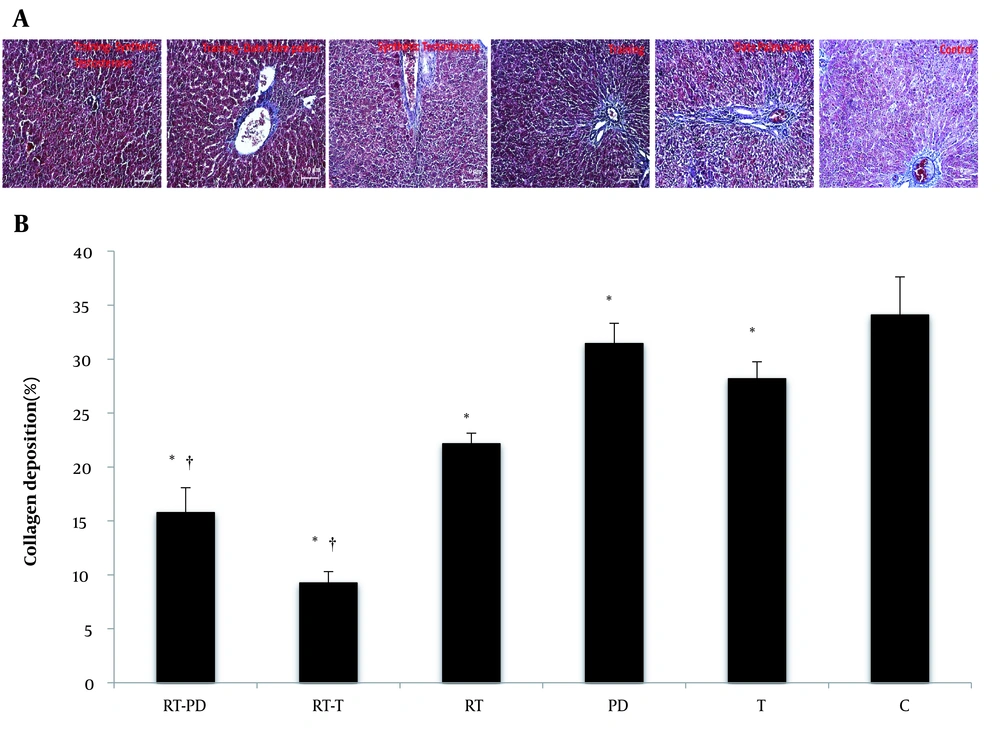
In this study, RT significantly reduced the percentage of collagen degradation (F = 499.56, P = 0.001, η = 0.943). Receiving drugs also had a significant effect on the percentage of collagen degradation (F = 61.27, P = 0.001, η = 0.803). Moreover, DPPE (P = 0.001 and synthetic T (P = 0.001) significantly reduced collagen degradation. The interaction of RT and DPPE also significantly reduced the percentage of collagen degradation (F = 8.46, P = 0.001, η = 0.361). Simultaneous RT and DPPE or synthetic T enhanced the effect of each other on reducing collagen degradation. However, when synthetic T was accompanied by exercise, the percentage of reduction in collagen degradation was higher than when exercise was with DPPE (Figure 1).
Percentage of collagen degradation in the studied groups (A and B). RT-PD, resistance training-Phoenix dactylifera; RT-T, resistance training-testosterone enanthate; Rt, resistance training; PD, Phoenix dactylifera; T, testosterone enanthate; C, control. * Denotes significant differences from the C group. † Denotes significant interaction. Data are expressed as mean and standard deviation.
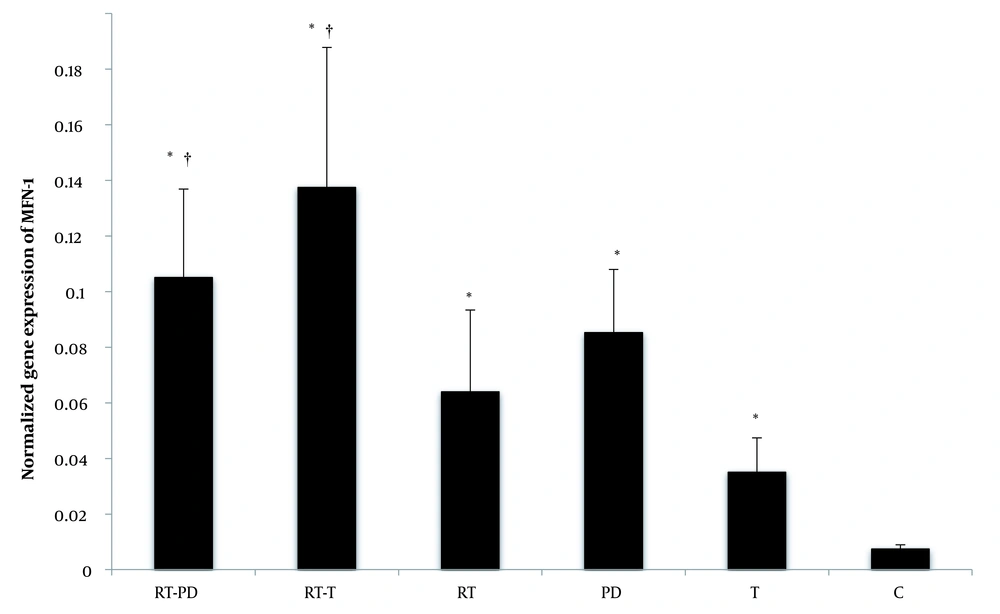
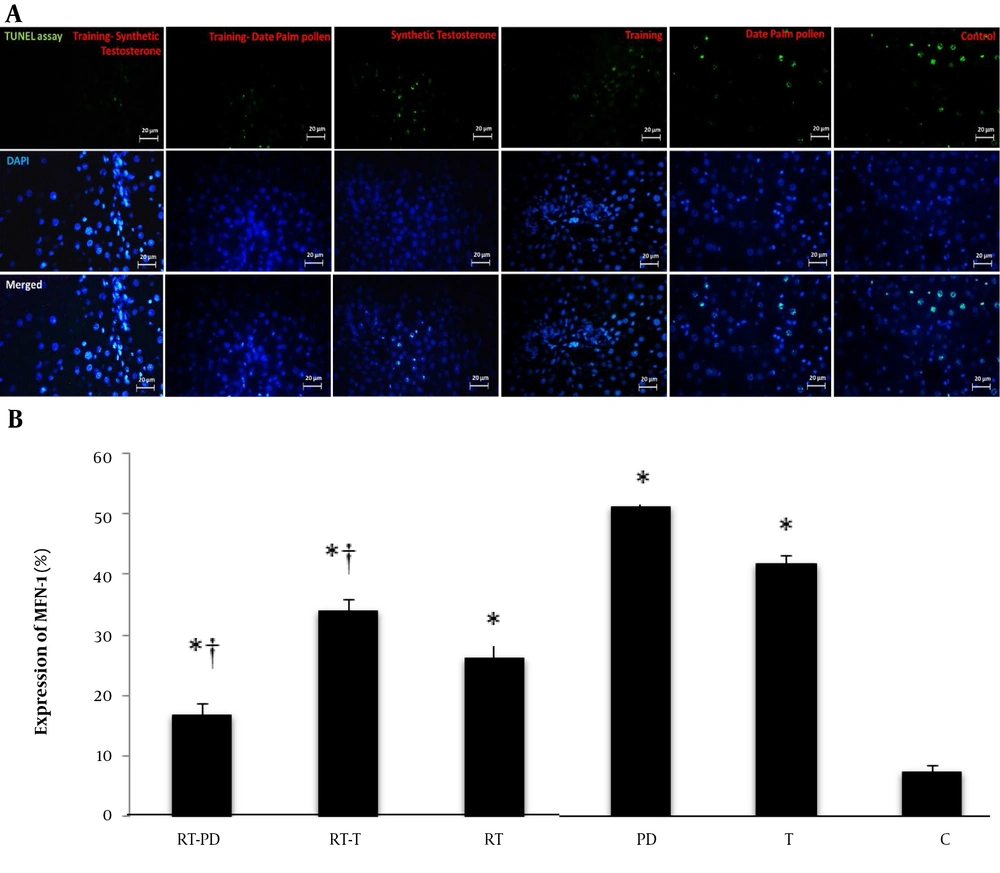
The RT significantly increased MFN-1 gene expression. (F = 37.56, P = 0.001, η = 0.556). Receiving the drug (synthetic T and DPPE) also significantly affected the MFN-1 gene expression (F = 14.53, P = 0.001, η = 0.492). So, DPPE (P = 0.001) and synthetic T (P = 0.001) might increase the expression of this gene. Also, RT and DPPE interaction caused a significant increase in MFN-1 gene expression (F = 6.04, P = 0.006, η = 0.287). In other words, RT, DPPE, and synthetic T might enhance the effect of each other on the expression of this gene. However, the effect of synthetic T was greater than that of DPPE (Figure 2).
MFN-1 gene expression in the studied groups. RT-PD, resistance training-Phoenix dactylifera; RT-T, resistance training-testosterone enanthate; Rt, resistance training; PD, Phoenix dactylifera; T, testosterone enanthate; C, control. * Denotes significant differences from the C group. † Denotes significant interaction. Data are expressed as mean and standard deviation.
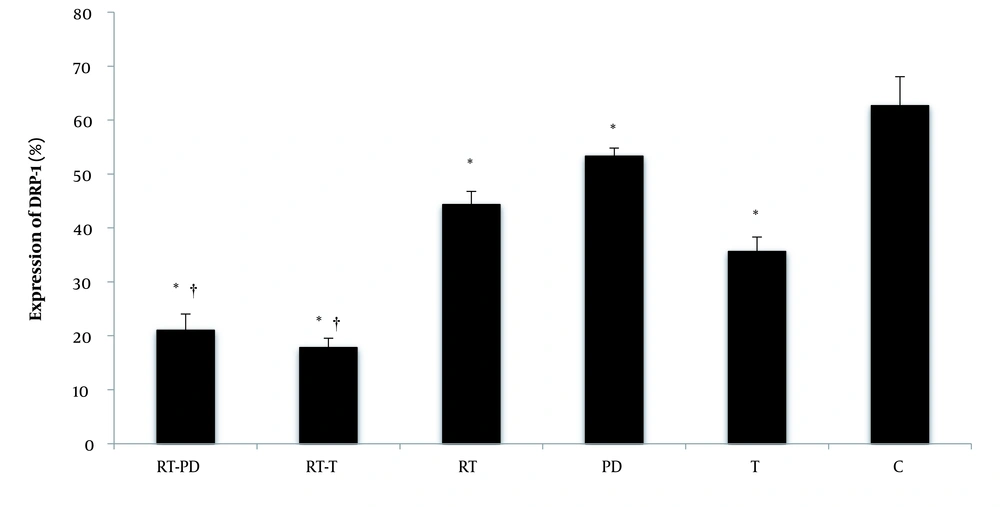
The RT significantly reduced DRP-1 protein expression (F = 270.77, P = 0.001, η = 0.900). Receiving drugs also had a significant effect on DRP-1 protein expression (F = 351.29, P = 0.001, η = 0.959). DRP-1 protein expression was significantly reduced by receiving DPPE supplements (P = 0.001 and synthetic T (P = 0.001). The interaction of RT and drug significantly reduced DRP-1 protein expression (F = 15.65, P = 0.001, η = 0.511). Simultaneous RT and DPPE or synthetic T enhanced the effect of each other on reducing DRP-1 protein expression. However, when synthetic testosterone was accompanied by training, the percentage of decreased DRP-1 protein expression was higher than the time when training was done with DPPE (Figure 3).
DRP-1 protein expression in the studied groups. RT-PD, resistance training-Phoenix dactylifera; RT-T, resistance training-testosterone enanthate; Rt, resistance training; PD, Phoenix dactylifera; T, testosterone enanthate; C, control. * Denotes significant differences from the C group. † Denotes significant interaction. Data are expressed as mean and standard deviation.
Moreover, RT significantly increased MFN-1 protein expression (F = 916.99, P = 0.001, η = 0.968). Receiving drugs also had a significant effect on MFN-1 protein expression (F = 372.42, P = 0.001, η = 0.961). In a way that both DPPE (P = 0.001) and synthetic T (P = 0.001) significantly increased the expression of this protein. The interaction of training and DPPE also significantly increased MFN-1 protein expression (F = 133.15, P = 0.001, η = 0.899). In other words, these two interventions were able to enhance the effect of each other on the expression of this protein. However, when synthetic testosterone was accompanied by training, the enhancement rate of this protein expression was higher than when training was done with DPPE (Figure 4).
MFN-1 protein expression in the studied groups. RT-PD, resistance training-Phoenix dactylifera; RT-T, resistance training-testosterone enanthate; Rt, resistance training; PD, Phoenix dactylifera; T, testosterone enanthate; C, control. * Denotes significant differences from the C group. † Denotes significant interaction. Data are expressed as mean and standard deviation.
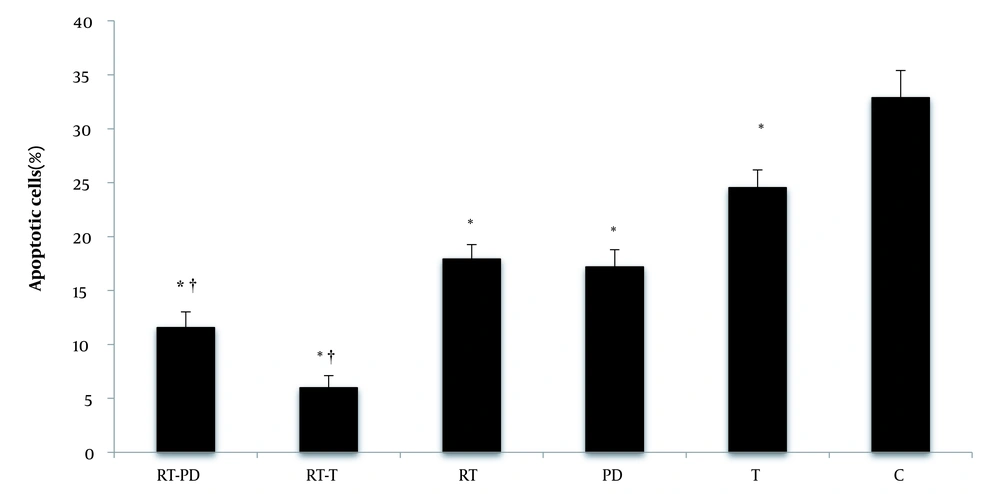
Training significantly reduced the percentage of apoptotic cells (F = 545.32, P = 0.001, η = 0.948). Receiving drugs also had a significant effect on the percentage of apoptotic cells (F = 160.37, P = 0.001, η = 0.914). Both DPPE (P = 0.001) and synthetic T (P = 0.001) significantly reduced the percentage of apoptotic cells. Also, the interaction of training and DPPE significantly reduced the percentage of apoptotic cells (F = 47.61, P = 0.001, η = 0.760). Simultaneous RT and DPPE or synthetic testosterone enhanced the effect of each other on the percentage of apoptotic cells. However, when synthetic testosterone was accompanied by exercise, the percentage of apoptotic cells was higher than when exercise was done with DPPE (Figure 5).
Percentage of apoptotic cells in the studied groups (A and B). RT-PD, resistance training-Phoenix dactylifera; RT-T, resistance training-testosterone enanthate; Rt, resistance training; PD, Phoenix dactylifera; T, testosterone enanthate; C, control. *Denotes significant differences from the C group. †Denotes significant interaction. Data are expressed as mean and standard deviation.
5. Discussion
Exogenous T increases athletic performance, but it is prohibited from most female and male competitions. Health risks can be produced by the long-term use of AAS and can result in several adverse events in different tissues and the incidence of drug-induced liver injury (DILI) (1, 6, 32). Consequently, finding herbs as natural supplements that can help the body make T, on the other hand, reduce the adverse health effects of T therapy in an athletic and older man, is of particular importance.
In the present study, we conducted a comparative evaluation between DPPE with T treatment alone and in combination with RT. Based on the results, combination therapy reduced the fission gene and protein expression levels in the liver compared to testosterone alone and DPPE alone treated groups. Furthermore, a combination of RT with T or DPPE reduced apoptosis and collagen deposition in the liver tissue.
Our results revealed that RT results in mitochondrial damage, apoptosis, and fibrosis in the liver. Indeed, RT includes performing low to moderate-intensity workouts for a long time. It has been shown to heighten muscle oxidation capacity and enhance mitochondrial biogenesis in skeletal muscle. In addition, RT activates mitochondrial stress by activating complexes I, IV, and V. Exercise has different effects on liver function. Sun and colleagues reported that exercise could cause oxidative and mitochondrial stress in the liver. They explained that exercise-induced oxidative and mitochondrial stress might be either damaging by causing injury or beneficial by activating defense systems (33). Mikami et al. showed that exercise increased the resting level of HSP70 in the liver and that HSP70 accumulation helps protect stress-loaded cells from damage caused by increased chaperone activity and suppression of apoptosis (34). Oxidative stress results in macromolecular damage to cellular DNA, proteins, and lipids. Reactive oxygen species (ROS) is one of the mitochondrial DNA-damaging agents and is known to be apoptosis-inducing.
Mitochondria are one of the essential ROS production sites. For example, eliminating fusion proteins, OPA1 or Mfns, causes mitochondrial fragmentation with increasing ROS levels. Fission protein knockdown (such as Fis1 and dynamin one protein (Drp1) does not affect ROS production in human bronchial epithelial cells while indicating mitochondrial fusion (35). Mitochondrial membrane potential reduction, defective ATP synthesis, and the overproduction of ROS are interrelated and lead to mitochondrial dysfunction (36). These factors are related to mitochondrial morphology changes. Li et al. reported that activation and overexpression of Drp1 exacerbated hepatic apoptosis (37). However, Zhang et al. revealed that activation of Drp1 remarkably diminished the apoptosis of hepatocytes (38). In contrast, in another research, it was shown that inhibition of Drp1 by small interfering RNAs could significantly improve the lost potential of the mitochondrial membrane in HT-22 cells (39). DRP1 activation and intracellular ROS increase are directly related to each other (40). Based on the cell’s metabolic needs, mitochondrial morphology is varied by regulated cycles of fusion (increases metabolic efficiency) and fission (favors uncoupled respiration to reduce oxidative stress) (41). Energy demand and stress induce the fusion of mitochondria. Studies have shown that Mfn2 preserves normal hepatic metabolism, and its erosion reduces mitochondrial respiration, decreases glucose tolerance, and increases hepatic glucose production (42). Therefore, our results were in line with previous studies that show a decrease in the expression of Mfn1 and Mfn2 genes and an increase in the Drp1 and Fis1 genes in RT.
Mitochondrial fission usually occurs under calm conditions, but it can also happen during high cellular stress levels when it activates apoptosis. Mitochondrial dynamics may play a significant role in liver fibrosis pathophysiology. It is demonstrated that hepatocyte epithelial-mesenchymal transition (EMT) is related to the mitochondrial dynamic imbalance induced by oxidative stress so that mitochondrial dynamics modulation can reverse liver fibrosis. Hepatic fibrosis occurs in many liver disorders and leads to scarring and liver damage (43-45). Liver fibrosis is a wound-healing response characterized by excessive deposition of extracellular matrix (ECM) proteins, hepatocyte damage, distortion of the hepatic lobules, and changes in vascular architecture. If left untreated, it can proceed to cirrhosis or liver carcinoma (46). It is supposed that exercises, probably via activating defense systems and a significant increase in enzyme activities, cope with the excess of free radicals produced by oxidative stress (33, 47).
Our results revealed that DPPE could reduce oxidative stress induced by RT, thus diminishing mitochondrial damage, apoptosis, and fibrosis in the liver. In line with our results, Saafi et al. showed the protective effect of date palm fruit extract (Phoenix dactylifera L.) on oxidative stress induced by dimethoate in rats’ livers (48). We supposed that the DPPE is likely to exert its beneficial effects by strengthening the immune system against exercise-induced oxidative stress.
5.1. Conclusions
The effects of a six-week RT program on mitochondrial mitofusin and mito-fission gene and protein expression and tissue damage in rats’ livers and the consequences of a combination of T and DPPE were investigated. The results showed that RT increased mitochondrial fragmentation in the liver. Treatment with the combination of DPPE and T caused expression elevation of the mitofusin gene and proteins. Also, apoptosis and collagen deposition in the liver were significantly reduced in combination therapy. These results suggest that DPPE is a natural compound that has characteristics like testosterone. With the ability to reduce the side effects of oxidative stress created during exercise in the liver and the positive impact in treating many diseases, it can be an excellent alternative to testosterone.