1. Background
Diabetes includes a group of metabolic diseases that occur due to hyperglycemia caused by some hormonal factors, such as defects in insulin secretion, function, or both (1). On the one hand, the type II diabetes (T2D) phenomenon occurs in response to insulin resistance in some tissues, such as skeletal muscle, liver, and adipose tissue. On the other hand, T2D occurs due to the inability of pancreatic β-cells to compensate for insulin resistance (2, 3). These defects occur as a result of both genetic and environmental factors (4) such that some of the recent studies have confirmed the direct effect of genetic disorders on reducing the function of β-cells, decreasing insulin secretion, and subsequently, the occurrence of T2D or increasing the T2D severity (5).
Meanwhile, the effect of glucose transporter 2 (GLUT2) in pancreatic cells is prominent on insulin secretion such that insulin is primarily synthesized and secreted in response to blood glucose or increasing the entry of glucose into β-cells (which is facilitated by GLUT2) (6). This is while other factors, such as free fatty acids and amino acids, lead to the acceleration of glucose-dependent insulin secretion (7). PDX1 has been found to inactivate the insulin gene and other glucose-sensitive genes that are effective in glucose metabolism, such as GLUT2 and glucokinase (GCK), in an adult pancreas (8, 9). On the other hand, in MafA-free mice, the expression of NeuroD, GLUT2, PDX1, and insulin decreases, and the glucose-dependent insulin secretion is severely damaged (10).
Glucose transporter 2, as the main transporter of glucose, is expressed in β-cells and makes possible the two-way flow of glucose and other food sugars, such as fructose and galactose, due to its high absorption capacity. Glucose transfer is the first step in glucose-dependent insulin secretion. A reduction in GLUT2 expression in human β-cells is associated with hyperglycemia and damage to glucose-dependent insulin secretion (11). Moreover, a direct relationship has been observed between the reduced glucose-dependent insulin secretion and the reduced expression of GLUT2 in β-cells in some animal species with T2D (11).
The available evidence shows that glucose and other nutrients act as the stimulators of GLP-1 secretion for insulin synthesis and release from the pancreas (12). Meanwhile, the response of other genetic or hormonal factors effective in insulin secretion to other external simulators, such as performing sports exercises with different methods, has been reported by some researchers (13-17), although the findings are more or less contradictory. For example, in a study by Lee et al., 12 weeks of low-intensity aerobic exercise led to a significant reduction in serum glucose and serum leptin levels, as well as a significant increase in GLP-1 in adolescent males with T2D (18). On the other hand, Eizadi et al. reported a decrease in transcription factor 7-like 2 (TCF7L2) expression as one of the other genetic factors affecting the insulin synthesis and secretion from pancreatic β-cells in response to long-term resistance training exercise in T2D rats (19), supporting the beneficial impact of sports activity on genetic factors affecting insulin secretion.
In spite of the available research findings, there is no study on the effect of sports exercise on the GLUT2 expression in pancreatic tissue of T2D rats. Therefore, considering the potential role of GLUT2 expression in insulin synthesis and secretion from pancreatic cells and the lack of a study in the literature on sports intervention in this regard, the present study was conducted with the aim of evaluating the effect of 10 weeks of aerobic and resistance exercise on GLUT2 expression in pancreatic tissue and on the fasting glucose levels and serum insulin in T2D male rats.
2. Objectives
The present study aimed to investigate the effect of aerobic and resistance exercise on GLUT2 expression in the pancreatic tissue and serum insulin of T2D male rats.
3. Methods
3.1. Ethical Considerations
The Ethics Committee of Islamic Azad University, Karaj Branch, Iran, approved the implementation of this study (code: IR.IAU.K.REC.1401.111).
3.2. Animals and Housing
The research population in this experimental basic study consisted of all Wistar male rats from the animal house of the Pasteur Institute in Iran. The research sample included 18 rats (age: 10 weeks, weight: 220 ± 10 g) that were selected randomly from the research population. The studied rats were kept in the laboratory environment in a 5 × 10 m2 room under controlled conditions of light (12-hour light and 12-hour dark, lighting starting at 6:00 p.m. and darkness starting at 6:00 a.m.), temperature (23 ± 3°C), and moisture (30 - 60%). Three rats were kept in Plexiglas cages (dimension: 25 × 27 × 43 cm3) with mesh doors such that they had ad libitum access to standard food and water. Two weeks after the rats were familiarized with the laboratory environment, T2D was induced by intraperitoneal infusion of nicotinamide (110 mg/kg of body weight) and freshly prepared streptozotocin (STZ) in citrate buffer (pH = 4.5) (60 mg/kg body weight) followed by a night fasting period of 12 hours (20). The healthy control group only received a citrate buffer with the same volume. To ensure diabetes induction in rats, the rats with fasting blood sugar above 15 mg/dL were considered diabetic one week later (21). The diabetes-induced rats were then randomly assigned into control, aerobic exercise, and resistance exercise groups.
3.3. Exercise Interventions
Aerobic exercises were performed based on Table 1 for 10 weeks, i.e., 5 sessions per week of treadmill running (22). All rats were dissected 48 hours after the last training session. The resistance exercises were also performed in accordance with Table 2 for 10 weeks, i.e., 5 sessions per week of stepladder climbing, 3 sets, 6 reps per set per session. The rest time between sets is 3 minutes and is 45 seconds per period between reps, which is modified from the Eizadi et al. protocol (19).
| Training Session (week) | 1 | 2 | 3 - 4 | 5 - 6 | 7 - 8 | 9 - 10 |
|---|---|---|---|---|---|---|
| Running time (min) | 10 | 20 | 30 | 40 | 50 | 55 |
| Running speed (m/min) | 18 | 20 | 22 | 22 | 24 | 26 |
| Training Session (week) | 1 | 2 | 3 - 4 | 5 - 6 | 7 - 8 | 9 - 10 |
|---|---|---|---|---|---|---|
| Resistance (body weight percentage) | 10 | 20 | 40 | 60 | 80 | 100 |
3.4. Blood Sampling and Tissue Extraction
Forty-eight hours after the last training session, following an overnight fast for 12 hours, blood sampling and tissue extraction were performed. The intraperitoneal infusion of a dosage of 50 mg/kg ketamine 10% and 10 mg/kg xylazine 2% was used to anesthetize rats. The animal's chest was then split open, and the blood sample was directly taken from the animal's heart to ensure the least harm to it. The samples were then taken from the rats' pancreas tissue and transferred to 1.8 microtubes containing 20% RNAlater solution for genetic tests after rinsing with physiological serum.
Using the glucose oxidase enzyme method and glucose kit (Pars Azmoun Company, Iran), the fasting glucose value was measured. Serum insulin was measured through the enzyme-linked immunosorbent assay (ELISA) method and according to the standards of the commercial kit (Demeditec Diagnostic insulin ELIZA) made in Germany. The ribonucleic acid (RNA) was extracted using the commercial RNeasy mini kit of QIAGEN Company (23). GLP mRNA was determined by real time-polymerase chain reaction (RT-PCR) using the Rotor-Gene 6000 system and One-Step SYBR TAKARA kit based on the company's instruction. The RNA polymerase II was used as the control gene to determine the expression of GLUT2. Table 3 shows the pattern of primer sequences. The cycle of thresholds (CTs) of the reactions were extracted by real-time-PCR software and recorded.
| Genes | Primer Sequence | Product Size | T m | Gene Bank |
|---|---|---|---|---|
| GLUT2 | For: GCATGTCTGTTACCCCAGGATAG | 159 bp | 60 | NM_001191052.1 |
| Rev: AGAGGAGTAACAAGCTCAAGGTG | ||||
| RNA PolymraseΙΙ | For: ACTTTGATGACGTGGAGGAGGAC | 164 bp | 60 | XM_008759265.1 |
| Rev: GTTGGCCTGCGGTCGTTC |
Abbreviation: RNA, ribonucleic acid.
3.5. Statistical Analysis
The obtained data were compared using one-way analysis of variance (ANOVA) and post hoc Tukey test with SPSS/Win software version 22.0. The Kolmogorov-Smirnov test was used to ensure the normal distribution of the data. A P-value of 5% was considered to be statistically significant.
4. Results
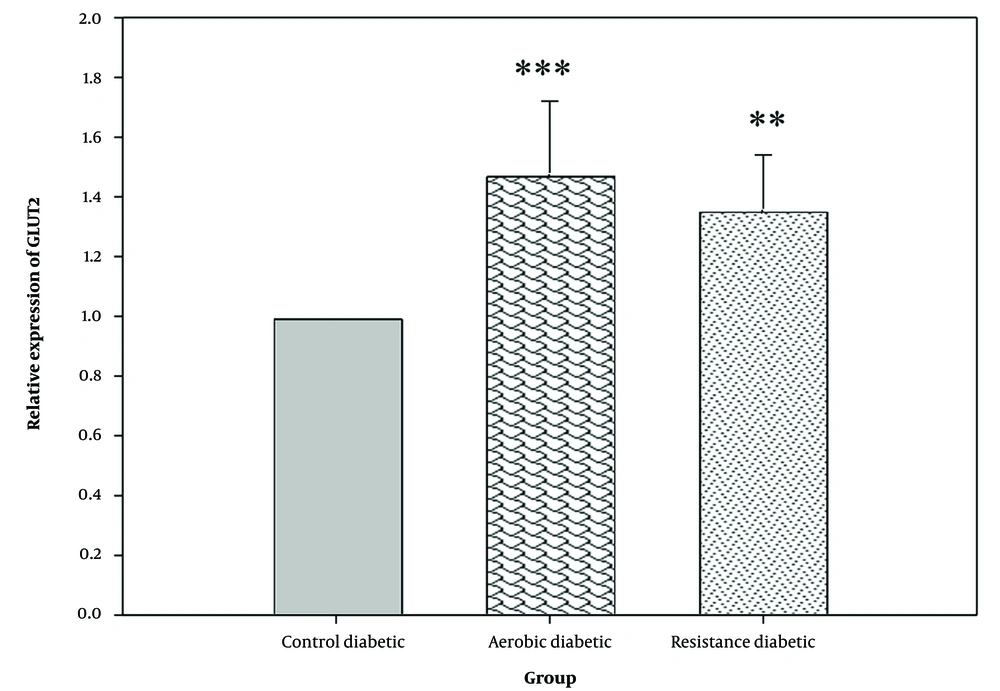
The one-way ANOVA results showed a significant difference in the expression of GLUT2 in pancreatic tissue between different groups (P = 0.001) (Figure 1). On the other hand, according to the findings of the post hoc Tukey test, both aerobic (P = 0.001) and resistance (P = 0.007) exercises led to a significant increase in GLUT2 expression as compared to the control group. However, no significant difference was observed in the expression of GLUT2 between the aerobic and resistance groups (P = 0.485).
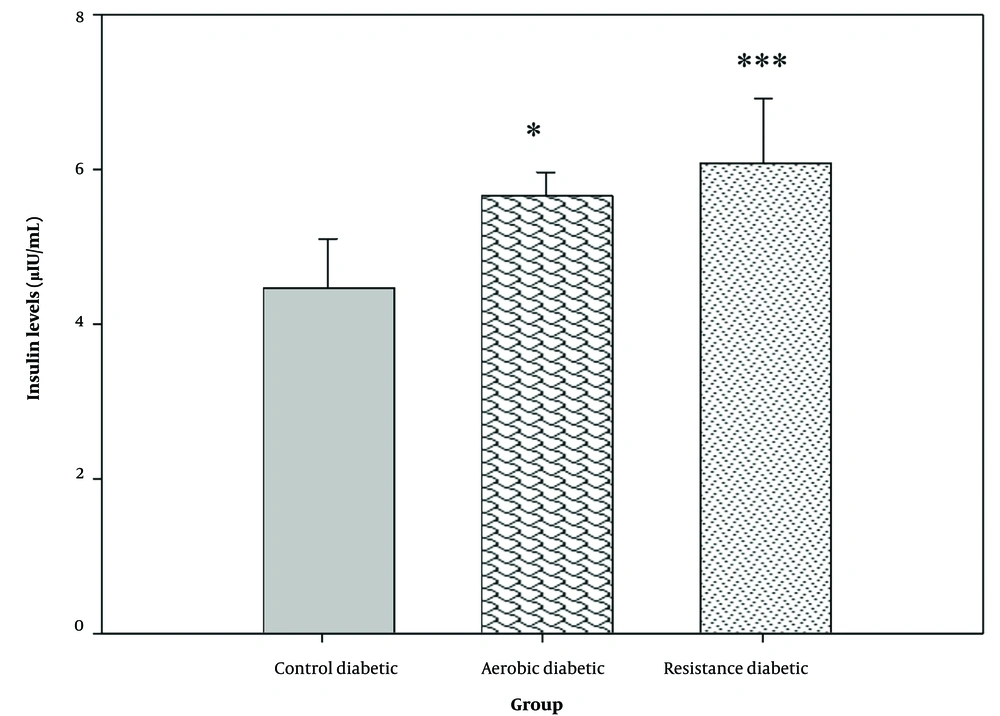
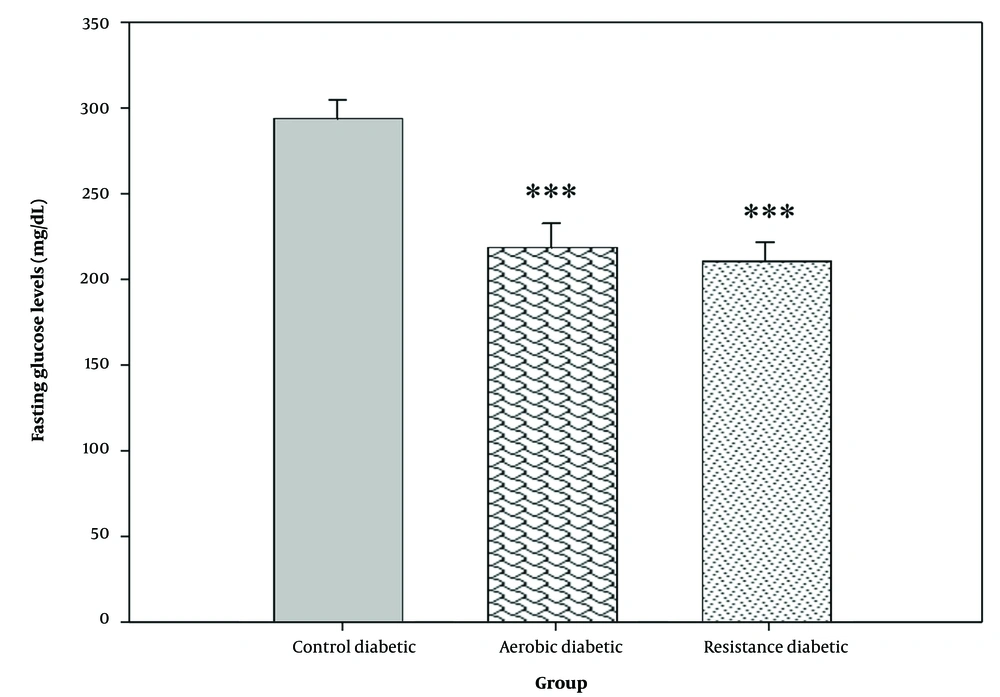
There was a significant difference in serum insulin and fasting glucose levels between the study groups (P = 0.001) (Figures 2 and 3). Both aerobic and resistance exercises caused a significant increase in serum insulin (P = 0.013 and P = 0.001, respectively) and a significant reduction in fasting blood glucose levels (P = 0.001 and P = 0.001, respectively) as compared to the control group. However, no significant difference was observed in serum insulin (P = 0.480) and fasting blood glucose levels (P = 0.552) between the aerobic and resistance groups.
5. Discussion
The main finding of the present study was the increased gene expression level of GLUT2 in the pancreatic tissue of T2D rats in response to training exercise interventions. In other words, 10 weeks of aerobic and resistance exercises led to a significant increase in the gene expression level of GLUT2 in the pancreatic tissue of T2D rats as compared to the control group that did not receive training exercise interventions. On the other hand, a significant reduction in the fasting blood glucose levels and a significant increase in serum insulin levels in the experimental groups, compared to the control group, in response to training exercise interventions were among the other findings of the present study.
Although the effect of sports exercise on the gene expression level of GLUT2 in the pancreas of diabetic rats has not been reported in many studies, Rashet and Abdi observed a significant increase in the gene expression level of GLUT2 in response to aerobic exercises in rats whose pancreases had become inflamed and damaged by high-fat diet (23). Sokhanvardastjerdi et al. reported a significant increase in the GLUT2 expression level in obese diabetic rats in response to aerobic exercises (24). Moreover, Simoes et al. showed an improvement in the function of insulin and insulin receptors and in the gene expression level of GLUT2 in response to aerobic exercises (25). However, to date, no study has reported the effect of resistance exercises on the expression level of GLUT2 in the pancreatic tissue of diabetic rats by comparing aerobic and resistance exercises.
On the other hand, several studies have been conducted with the aim of evaluating the effect of exercise training interventions on blood glucose levels, serum insulin, insulin resistance, and hormonal or genetic factors affecting these variables in T2D individuals and other healthy or unhealthy populations. In this regard, some studies have reported a reduction in insulin secretion following sports exercises. For example, in a study by Rawal et al., 12 weeks of aerobic exercise led to a reduction in insulin levels in healthy males (26); however, in another study on diabetic rats, long-term exercise increased the plasma insulin levels by 57%, compared to the control group (27). A significant elevation in the plasma insulin levels was also observed in diabetic rats following 5 weeks of sports exercise (28).
However, in line with the results of the present study, all of the above-mentioned studies reported a significant reduction in blood glucose levels. According to the aforementioned evidence, the researchers concluded that the response to glucose as insulin synthesis or secretion and β-cells function in human or animal species are different from each other depending on the presence or absence of diabetes, diabetes severity, the age of diabetes induction in the animal, and the age of entry to exercise, which affect the findings to some extent (26, 29). The researchers believe that most of the beneficial effects of sport and physical activity on blood glucose levels in healthy non-diabetic patients, such as obese individuals, become evident due to the reduction of the insulin resistance of peripheral tissues, especially skeletal muscles (30).
Plasma glucose levels are tightly regulated by the simultaneous action of insulin and glucagon as two hormones secreted by the pancreas, which have conflicting effects on the glycemic profile (12). Patients with T2D often experience a decline in β-cells function, resulting in reduced insulin secretion from these cells. On the other hand, scientific resources have shown that T2D occurs in response to both a reduction in β-cells function and an increase in insulin resistance (31), although an increase in the glucagon-dependent glucose release from the liver reservoirs and rapid increase in glucose absorption from food are among other factors that increase blood glucose levels in these patients (31). Meanwhile, the impact of some genetic or hormonal factors affecting insulin synthesis, secretion, and function in target cells should not be overlooked.
Among important therapeutic factors in T2D, GLUT2 can be referred to (11). Although insulin is secreted due to some stimulants, such as nutritional stimulants (amino acids, e.g., leucine, unsaturated fatty acids, and glutamine combined with leucine), hormones, neurotransmitters, and drugs (e.g., sulphonylureas and glinides), glucose is the main physiological stimulus of insulin secretion (32). According to some of the accepted hypotheses, insulin secretion is a multi-step process initiated by transferring glucose into β-cells through particular transporters, especially GLUT1 and GLUT2, and phosphorylation by glucokinase that charges the flow of glycolysis, with pyruvate as its final product (33). In this regard, a kind of strong correlation has been reported between the declined function of β-cells and the reduced expression level of GLUT2 as a glucose transporter in β-cells (34, 35).
Clinical studies found GLUT2 essential for glucose-dependent insulin secretion and stated that the absence of GLUT2 is accompanied by hyperglycemia. Transferring glucose into β-cells is the first step in the relationship between glucose metabolism and insulin secretion from β-cells. GLUT2 is considered the main transporter of glucose in β-cells that allows the two-way flow of glucose and other food sugars, such as fructose and galactose, due to its high adsorption capacity. Transferring glucose is the first step in the glucose-dependent insulin secretion. A reduction in the gene expression level of GLUT2 in human β-cells is associated with hyperglycemia and damage to glucose-dependent insulin secretion (11).
Additionally, a direct relationship has been observed between the reduced glucose-dependent insulin secretion and the reduced expression of GLUT2 in β-cells in some animal species with T2D (11). Hou et al. (36) reported that the high extracellular glucose concentrations increase the endocytosis GLUT2, leading to insulin secretion in parallel to an increase in the gene expression level of GLUT2 (36, 37) such that the initial phase of glucose-dependent insulin secretion cannot be observed in β-cells in the GLUT2-deficient mice (11). A cellular-molecular study by Koranyi et al. showed that 3 weeks of aerobic exercise led to a reduction in the mRNA pro-insulin and mRNA glucokinase content but caused no change in the content of mRNA GLUt2 as the main transporter of glucose. On the other hand, a direct relationship was observed between a reduction in the mRNA pro-insulin content and a decline in mRNA glucokinase (38).
In summary, the genetic studies strongly support the influential effect of GLUT2 on the pathways leading to insulin synthesis and secretion in β-cells. Although aerobic and resistance exercises were not exactly the same in this study, the findings of the present study showed an increase in the GLUT2 expression level in response to both resistance and aerobic exercises in T2D rats. As no significant difference was observed between the effects of these two exercise methods, sports exercises seem to affect the expression of GLUT2 in the pancreas of diabetic rats independent of the exercise type.
5.1. Conclusions
Performing aerobic and resistance exercises is accompanied by an increase in serum insulin levels and a decrease in blood fasting glucose levels in T2D rats. Considering the effective role of GLUT2 in the process of insulin synthesis and secretion, the increased insulin levels can probably be attributed to the elevated expression levels of GLUT2 in pancreatic tissue in response to resistance and aerobic exercises. Sports exercises seem to affect the expression of GLUT2 in the pancreas of diabetic rats independent of the exercise type.