1. Background
Neural stem cells (NSCs) can self-renew and differentiate into a variety of cells, including neuronal and glial cells, in both in vitro and in vivo conditions (1). The ability of endogenous NSCs for neurogenesis and gliogenesis in the embryonic and adult brain has been demonstrated in response to inflammation, ischemic conditions, and traumatic events (2, 3). The potential for repair and replacement of cells in the adult brain through endogenous neurogenesis and gliogenesis supports the development of therapeutic approaches involving NSC transplantation in brain disorders. However, the necessity of expanding and pre-differentiating NSCs before administration into the injured site of the brain should be considered (2). Neural stem cells from embryonic tissue of rodents can be isolated and cultured in serum-free conditions (4). Neurogenesis plays an essential role in the development and plasticity of the central nervous system (CNS) (5, 6). It involves two important stages: Proliferation and differentiation (7), which are regulated by several intrinsic and environmental factors. Stem cells are multipotent, and the substitution of proliferative culture medium with differentiative conditions forces NSCs to differentiate into neuronal and glial cells (8, 9).
Curcumin, a polyphenol component, was isolated from the rhizomes of Curcuma longa L. (turmeric) two centuries ago (10). This component has many therapeutic effects (11), including anti-inflammatory, anti-oxidative, and anti-cancer properties (12). Experimental analyses in animal models have shown that curcumin has beneficial effects on a wide variety of neurodegenerative diseases (11, 13). Some interesting studies have focused on evaluating curcumin's effects on neurogenesis (8, 14). Neuronal stem/progenitor cells of the hippocampus are regulated by intrinsic and extrinsic factors in both positive and negative ways (15). Unfortunately, these studies primarily concentrated on the proliferative effects of curcumin and did not consider its regulatory role in neurogenesis.
2. Objectives
In the current study, both the proliferation and neurogenesis of embryonic rat NSCs were considered. Therefore, the influence of curcumin on the proliferation and differentiation of NSCs was examined. Furthermore, the neurospheres were characterized by the expression of nestin, class III β-tubulin (Tuj-1), and glial fibrillary acidic protein (GFAP) markers.
3. Methods
3.1. Animals
Female Wistar rats, weighing 180 - 200 g, were housed in normal room conditions at a constant temperature (25ºC) with a 12-hour light/dark cycle and ample access to food and water. The experiments were approved by the ethics committee of animal research at Islamic Azad University, Science and Research Branch, Tehran, Iran, under ethical approval number IR.MUQ.REC.1397.119.
3.2. Experimental Design
At the beginning of the experiment, there were 50 female rats and 50 male rats. Male and female rats were kept together in a cage overnight for mating. The next day, vaginal plugs were regularly screened to determine pregnancy. If a vaginal plug was observed, it was considered the zero-day of pregnancy (E = 0). Pregnant rats (n = 36) at gestational age 15.5 days (E15.5) were then sacrificed by intraperitoneal injection (i.p.) of an overdose of sodium pentobarbitone, and the fetuses (n = 176) were extracted. The brains of female fetuses (n = 50) were then dissected and prepared for NSC extraction.
3.3. Neurosphere Culture
Neurospheres were cultured using previously described methods with minor modifications (16, 17). After removing the overlying meninges and blood vessels from the isolated fetuses’ brains, the sub-ventricular zone (SVZ) of each head was isolated and transferred to serum-free media. The cultures were incubated at 37°C in a humidified atmosphere with 5% CO2. The cultures were assigned into four experimental groups as follows: (1) control: No treatment, (2) (exposure to 0.1 µM curcumin), (3) (exposure to 0.5 µM curcumin), and (4) (exposure to 1 µM curcumin). For differentiation studies, after four days of in vitro culture, the counted cells were seeded onto Poly-L-lysine (Sigma-Aldrich)-coated surfaces and developed into a monolayer in the same mitogen-free medium.
3.4. The MTT Assay: Cell Viability
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma] method (18) was employed to evaluate cell growth and viability based on the reduction of MTT into formazan crystals by mitochondrial dehydrogenase enzymes of viable cells. The absorbance of the samples was read at 570 nm. All experiments were replicated at least three times to reduce probable errors.
3.5. Immunocytochemistry (ICC)
After four days in vitro (DIV), the cells were processed by ICC to evaluate the expression of the antigen and for morphology-related analysis. Subsequently, the nuclei were counterstained with propidium iodide (1/15000, Sigma-Aldrich).
3.6. Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
The neurospheres were identified by the expression of nestin, class III β-tubulin (Tuj-1), and GFAP. Total RNA was extracted using TRIZOL reagent, and cDNA synthesis and PCR were carried out using a one-step RT-PCR kit (Invitrogen). The samples were evaluated on a 1% agarose gel containing ethidium bromide.
3.7. Morphometric Analysis
Four days after the proliferation condition, five non-overlapping fields were randomly chosen from each well. Digital images of the neurosphere cultures were taken using an inverted microscope. Finally, ImageJ software (version 1.53t 24) was used to analyze the size of the neurospheres.
3.8. Statistical Analysis
Our findings are presented as the mean ± standard error of the mean (SEM). Statistical analysis was carried out using one-way ANOVA and Tukey’s post-hoc test. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Characterization of Neural Stem Cells
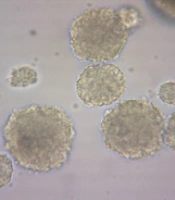
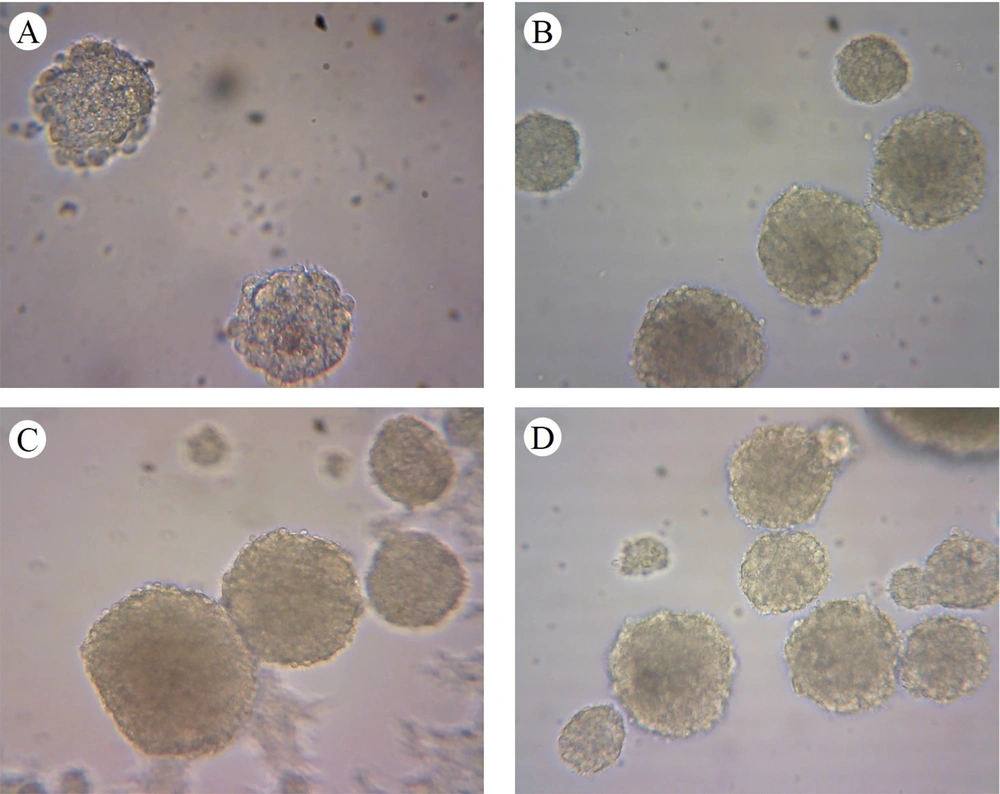
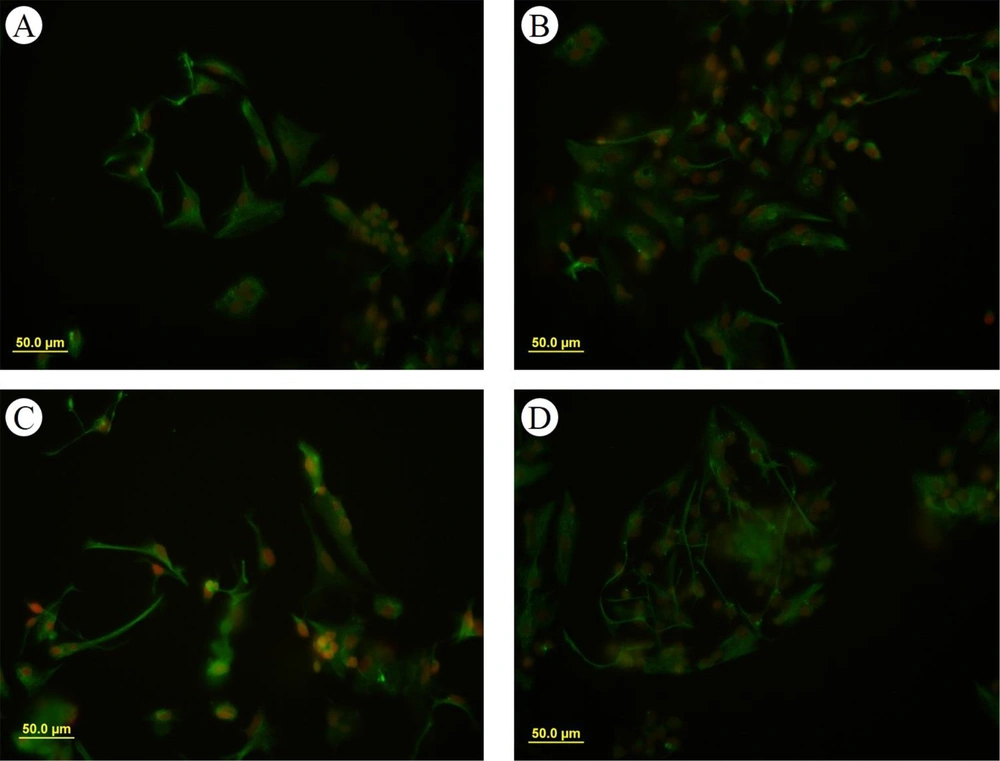
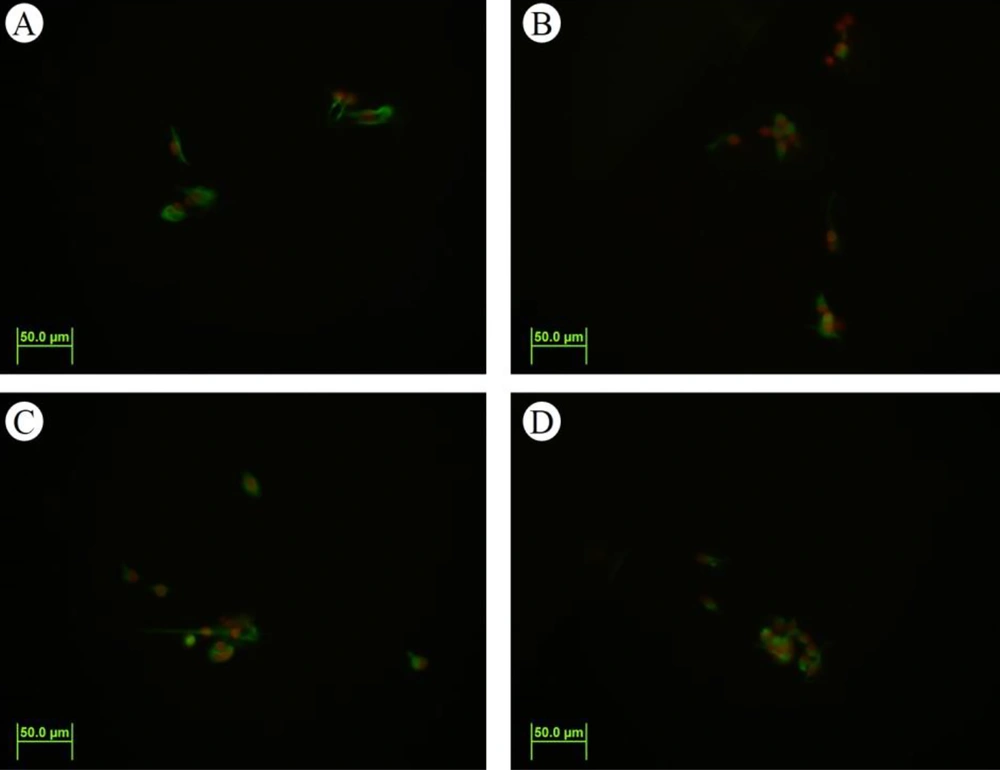
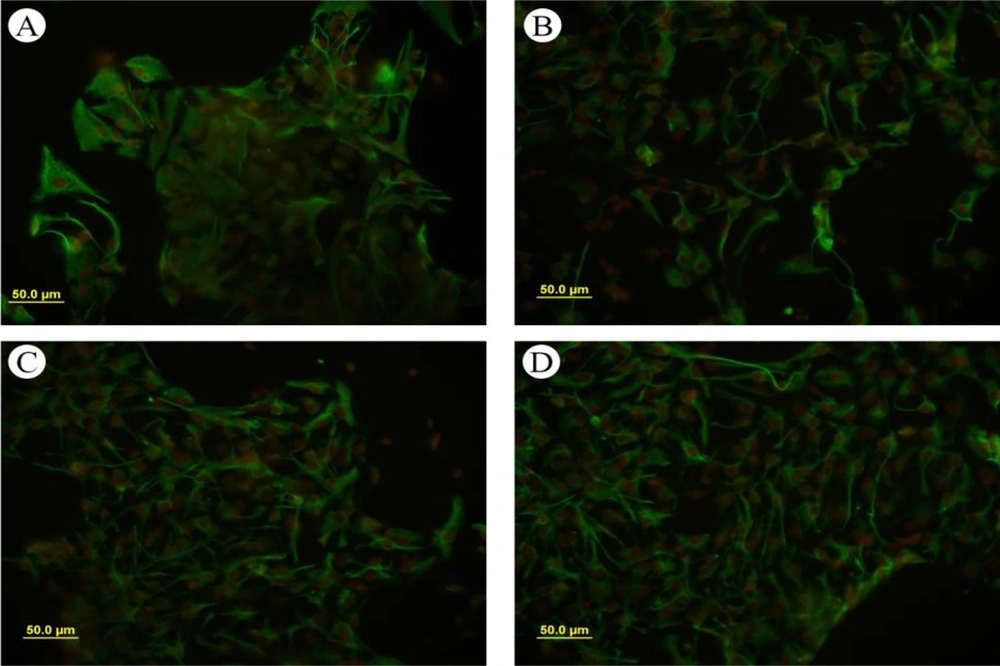
The formation of neurospheres is a marker of stemness (Figure 1A). When these neurospheres were dissociated into single cells, they were cultured into a monolayer and subsequently immunostained with the nestin marker, showing nestin positivity (Figure 2). The substitution of the proliferative medium with the differentiation medium was executed by withdrawing growth factors and adding fetal bovine serum (FBS) to the medium, resulting in the differentiation of the cultured cells. Immunocytochemistry analysis for the presence of Tuj-1 and GFAP proteins showed positive results (Figures 3 and 4A). The cultured cells exhibited characteristics of NSCs, demonstrating self-renewal and multipotency. Tuj-1 immunoflurescent staining is given in Figure 4. The control group shows apoptotic cells and the lowest amount of NSCs (Figure 4A). On the other hand, treatment with curcumin at doses 0.1 µM (Figure 4B) and 0.5 µM (Figure 4C) could successfully increase the number of NSCs. It should be noted that curcumin at high dose of 1µM was not that much effective (Figure 4D).
4.2. Effects of Curcumin on Diameter of Neural Stem Cells
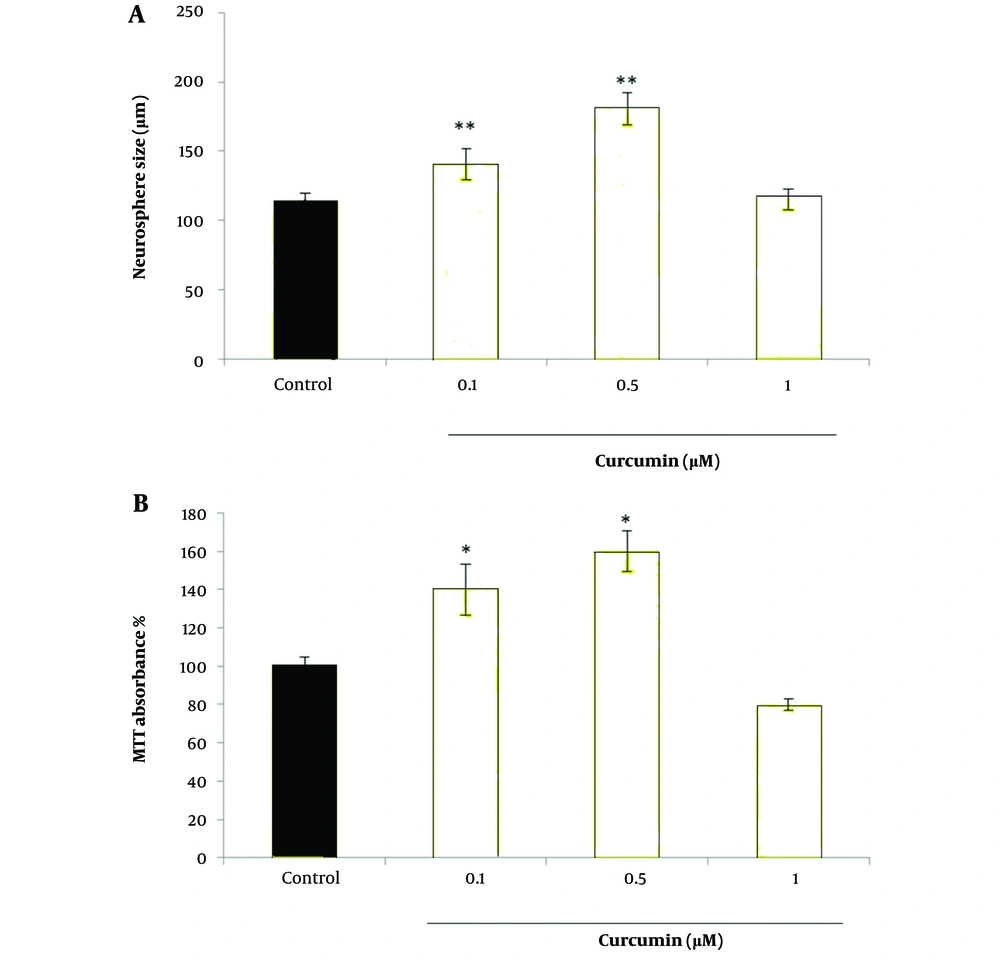
Evaluating the diameter of cultured neurospheres is a proper method for assessing the proliferation rate of NSCs. The size of spheres from the primary culture was measured after 8 days. Simultaneously, images were taken from various fields, and the size of the neurospheres in each field was evaluated. Curcumin at doses of 0.1 µM and 0.5 µM increased the size of neurospheres (P < 0.05) (Figures 1 and 2).
4.3. Effect of Curcumin on Viability of the Neural Stem Cells
The effects of curcumin on neurospher size are given in Figure 5. The control group (Figure 5A) shows the lowest expression of Nestin and GFAP. On the other hand, treatment with curcumin at doses 0.1 µM (Figure 5B) and especially at dose 0.5 µM (Figure 5C) could successfully increase the expression levels of the genes. It should be noted that curcumin at high dose of 1µM was not that much effective (Figure 5D). Cell viability was determined using the MTT reduction assay. Figure 5B demonstrates the MTT results of the neurospheres. The viability of cells exposed to curcumin at doses of 0.1 µM and 0.5 µM was markedly increased compared to the control group (P < 0.05).
4.4. Effects of Curcumin on Expression of Neural and Glial Markers in Neural Stem Cells
The NSCs could express markers of glial and neural cell differentiation under specific differential conditions. Notably, morphological studies revealed that the GFAP-positive cells obtained from cultures treated with different doses of curcumin exhibited obvious morphological changes. The GFAP-positive cells in the curcumin groups were long and thin, while the GFAP-positive cells in the control groups appeared to be polygonal and flat (Figure 3).
4.5. Effects of Curcumin on Nestin, Glial fibrillary acidic protein and Tuj-1 m RNA Expressions
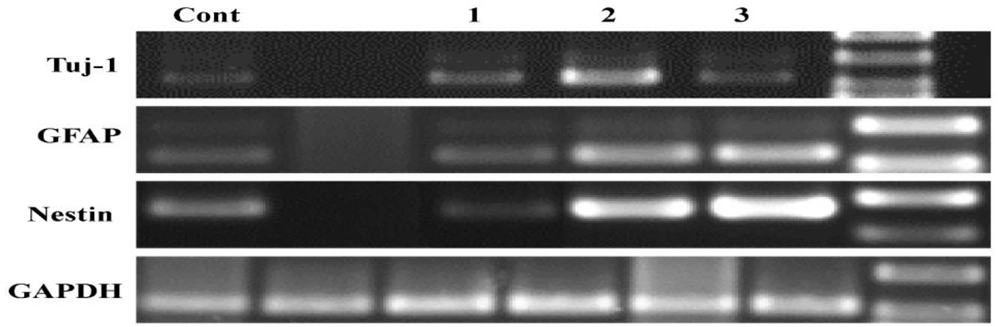
The expression levels of nestin, GFAP, and Tuj1 were measured in the neurospheres treated with curcumin. As shown in Figure 6, nestin expression levels were increased by 0.1 and 0.5 µM of curcumin. In addition, GFAP expression was similarly increased. However, Tuj1 expression levels increased in the group treated with 1 µM of curcumin (Figure 6).
5. Discussion
In this study, curcumin was found to stimulate the proliferation of embryonic NSCs at low doses (0.1 and 0.5 µM) using the MTT assay, with the highest level of proliferation observed at a dose of 0.5 µM. Conversely, proliferation declined at a high dose of curcumin (1 µM). Furthermore, NSCs exposed to different doses of curcumin exhibited a biphasic pattern of proliferation. Reverse transcription polymerase chain PCR reaction analysis showed that nestin and GFAP expressions reached their highest levels in curcumin-treated cells at a dose of 0.5 µM, while Tuj-1 expression levels increased in curcumin-treated cells at 1 µM.
Mitogenic growth factors play an important role in establishing neurosphere culture and are necessary for the growth and viability of free-floating neurospheres (8, 19). In this study, we verified that supplementing the proliferation condition with curcumin can enhance the size of neurospheres that were only supplemented with mitogenic factors. The simultaneous addition of curcumin and growth factors induced the growth of neurospheres. Similar to the MTT assay, a higher diameter of neurospheres was observed with 0.5 µM of curcumin.
In addition, the differentiation of NSCs in this study was assessed by detecting Tuj-1 and GFAP markers. Immunocytochemistry results indicated that in the presence of curcumin, the NSCs differentiated into neurons and glia. Reverse transcription polymerase chain reaction PCR analysis revealed that higher doses of curcumin corresponded to higher expression levels of Tuj-1. Under differentiation conditions, especially in the presence of FBS, a greater proportion of cells differentiated into astrocytes, and only a small portion of stem cells followed a neuronal fate (< 5%) (20).
In line with our experiment, curcumin promoted the differentiation of glioma-initiating cells (GICs). Curcumin (2 μM) stimulated GIC differentiation and inhibited glioma growth, which is related to the induction of autophagy in vitro and in vivo (21). Additionally, the effects of curcumin (1 or 5 μM) on oligodendrocyte progenitors (OPs) were recently assessed. Curcumin promoted the differentiation of OPs and counteracted the maturation arrest of OPs induced by TNF-α (22).
Consistent with our experiment, the impacts of curcumin on mouse multipotent neural progenitor cells (NPCs) and mature hippocampal neurogenesis were shown. Curcumin exhibited a biphasic response on cultured NPCs; low concentrations (0.1, 0.5 μM) stimulated cell proliferation, whereas high concentrations (≥ 10 μM) were cytotoxic. Moreover, curcumin induced the proliferation and differentiation of cultured NSCs and encouraged neurogenesis in the healthy mature hippocampus (8). In another study, NPCs and cultured neurons exposed to celecoxib were examined. Curcumin attenuated celecoxib-induced inhibition of neurogenesis in the fetal frontal cortex via the Wnt/β-catenin pathway (23). Similarly, following cerebral ischemia in mice, curcumin stimulated neurogenesis in the hippocampal dentate gyrus via the Wnt/β-catenin signaling pathway (24). Recently, neonatal curcumin treatment (20 mg/kg, i.p.) restored hippocampal neurogenesis and improved autism-related symptoms in an experimental mouse model of autism (25).
Furthermore, in vitro treatment of cell cultures and in vivo treatment of adult rodents with curcumin protected neurons from damage related to Alzheimer's disease (AD), Parkinson's disease (PD), and stroke models (26-28). Curcumin improved cognitive functions in different neuropathologic models such as diabetic encephalopathy and ischemia (29). Animal model studies have suggested that curcumin is neuroprotective in neurodegenerative disorders such as AD (30, 31) and focal cerebral ischemia (32). Additionally, curcumin treatment protected hippocampal neurons against excitotoxic and traumatic injury (26, 33). Recently, in a rat model of AD, curcumin treatment repaired cognitive impairments and enhanced hippocampal neurogenesis. Curcumin dose-dependently (50 and 100 mg/kg/d, i.p.) increased the proliferation of NSCs, stimulated differentiation, and maturation of newly generated neural cells, and increased the expression of neurogenesis-involved proteins (34).
Many studies have demonstrated the proliferative role of curcumin on neuroprogenitor cells (8, 35, 36). Previous studies have documented the antioxidant and anti-inflammatory effects of micromolar concentrations of curcumin in cultured tumor cell lines as well as normal cells (37, 38).
5.1. Conclusions
Curcumin regulated both the proliferation and differentiation steps of neurogenesis in embryonic hippocampal NSCs. Treatment with curcumin could provide an alternative method for the pre-differentiation of NSCs before cell replacement therapy. The regulatory role of curcumin on the fate of NSCs was noted in the present study. It has been shown that curcumin may alleviate cognitive deficits resulting from various circumstances, such as aging and brain ischemia, by promoting the proliferation and neuronal differentiation of NSCs.