1. Context
Pesticide is defined as any compound or mixture of chemicals designed to prevent, eliminate, or control pests, encompassing both chemical and biological agents (1, 2). Pesticides are categorized into four primary groups based on their chemical structure: Organochlorines (e.g., DDT, methoxychlor, dieldrin, chlordane), organophosphorus (e.g., parathion, chlorpyrifos, diazinon), carbamates, and pyrethrin and pyrethroids (3). Despite attempts to restrict their application, pesticides are extensively utilized in the developing world. Concerns have been expressed regarding the safety of prolonged pesticide usage (4). Pesticide exposure may occur directly through occupational, agricultural, and domestic applications, as well as indirectly via dietary consumption (4). The primary pathways of human exposure to pesticides include the food chain, air, water, soil, vegetation, and animals (5).
Pesticides are typically disseminated within the organism by associating with plasma proteins, blood cells, and lipids in different body parts and peripheral tissues (2). Pesticide exposure is associated with a range of adverse health consequences, ranging from moderate irritation of the eyes and skin to serious complications, including neurodegenerative diseases (NDs) (6). Neurodegenerative illnesses are defined by disease-selective features of adult-onset neuronal degeneration within sections of the brain's cortex, basal ganglia, cerebellum, brainstem, and neuromuscular systems (7). Common examples of neurodegenerative disorders are Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease, amyotrophic lateral sclerosis, frontotemporal dementia, and spinocerebellar ataxias (8). These disorders impair numerous aspects of human functioning and impede the capacity to accomplish both basic (e.g., speech, movement, stability, and posture) and complex tasks (e.g., urinary and bowel functions, and mental abilities) (9).
A study reported that the risk of AD is higher in areas of high pesticide exposure (10). Furthermore, exposure to pesticides was associated with faster PD symptom progression (11). Most previous studies focused on the adverse effects of pesticides and the incidence of NDs. However, little is known about the mechanistic association between pesticides and NDs. Considering the importance of environmental factors in the etiology of NDs and the increase in the incidence of NDs (AD and PD) in recent years, we conducted a mini-review to fill the identified research gap and understand the biological mechanisms behind pesticide exposure and NDs.
2. Evidence Acquisition
Scientific literature was searched for pesticide exposure and NDs, focusing on biological mechanisms between 2000 and 2024. Articles were retrieved from PubMed, Scopus, Web of Science, and Google Scholar databases using the following keywords: Pesticides, pesticide exposure, organophosphates, in combination with neurodegenerative, PD, and AD. Subsequently, two independent researchers screened the retrieved articles.
3. Results
3.1. Alzheimer's Disease
3.1.1. Progression of Alzheimer's Disease on Exposure to Pesticides
Pesticides, recognized as toxic agents, pose significant risks to various bodily systems in humans, particularly the central nervous system (CNS). Their detrimental effects manifest through multiple mechanisms, including the inhibition of neurotransmitter receptors, disruption of transport channels, and mitochondrial damage, all of which can lead to heightened oxidative stress. These physiological alterations may impair motor, sensory, autonomic, and cognitive functions (12-14). In our research, we investigated the effects of pesticides on the activation of tau protein and beta-amyloid formation, both of which are critical pathophysiological factors contributing to synaptic and neuronal loss, ultimately leading to AD.
3.1.1.1. Pesticides and Tau Protein
Tau protein is a type of microtubule-associated protein (MAP) predominantly located in the cytoplasm and axonal structures of neurons (15). Exposure to pesticides may induce morphological changes in the CNS due to the phosphorylation of tau protein and the formation of neurofibrils (16, 17). Different studies have reported mechanisms underlying tau protein formation caused by pesticides. For instance, one study reported that pesticides could lead to the formation of tau protein through polymorphisms in the microtubule-associated protein tau (MAPT) and MAP 1B gene (18). Another study demonstrated that organochlorines could alter mitochondrial function and lead to tau protein formation, exhibiting characteristics of AD, with overexpression of specific proteins, including cytochrome C, synaptosome-associated protein, and enclose A (19, 20).
In addition to the overstimulation of glial cells, elevated levels of tumor necrosis factor-alpha, interleukin-6, and interleukin-1 beta have been implicated in the increased presence of hyperphosphorylated tau protein observed following exposure to organophosphates (OP) (21, 22). Significant epidemiological associations have been identified between pesticide exposure and AD, particularly concerning the effects of OP in males (23). Carbamates represent another type of pesticide that can affect the nervous system of laboratory animals by inhibiting cholinesterase enzyme receptors (24). In another study, mice exposed to carbamates exhibited decreased levels of dopamine (25). As is well-known, a decline in dopamine-firing cells can impair the brain's ability to create new memories, particularly in regions like the hippocampus, and could lead to AD (26).
3.1.1.2. Pesticides and amyloid-beta
Amyloid beta (Aβ) is a peptide that plays a central role in the pathology of AD. Overexpression of this peptide can lead to the accumulation of Aβ peptides, specifically Aβ40 and Aβ42, resulting in neurotoxicity and ultimately causing neural cell death (27). Currently, the hypothesis that the deposition of β-sheets contributes to the development of AD is the most widely accepted among researchers (28). Pesticides can enhance the accumulation of Aβ through various mechanisms (16). For instance, a study showed that exposure to DDT significantly increases the amyloid precursor protein (APP) and mRNA levels in 3xTG-AD mice. Additionally, the study observed a loss of synaptic markers (29).
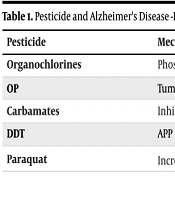
Another study demonstrated that exposure to the pesticide paraquat can lead to increased levels of Aβ protein in mice through mechanisms involving mitochondrial dysfunction (30). One mechanism through which certain pesticides may contribute to an increase in Aβ deposition, ultimately leading to AD, involves alterations in sex hormone levels, including estrogen and androgens (31, 32). Evidence from various studies indicates that sex hormones function as neuroprotective agents, and disruptions in their levels may exacerbate Aβ deposition, potentially leading to conditions such as AD in middle-aged individuals (33, 34). Pesticides and AD-related biological mechanisms are summarized in Table 1.
| Pesticide | Mechanisms |
|---|---|
| Organochlorines | Phosphorylation of tau protein |
| OP | Tumor necrosis factor-alpha, interleukin-6, and interleukin-1 |
| Carbamates | Inhibiting cholinesterase enzyme receptors decrease in levels of dopamine |
| DDT | APP |
| Paraquat | Increased levels of Aβ protein |
Pesticide and Alzheimer's Disease -Related Biological Mechanisms
3.2. Parkinson's Disease
Parkinson's disorder is the second most prevalent neurological disease after AD and affects approximately 2% of people over the age of 65 in industrialized countries (35). While the actual etiology of sporadic PD remains largely uncertain, both environmental variables and genetic susceptibility are believed to play significant roles in its onset (36). Autosomal dominant Parkinsonian syndromes have been linked to rare missense mutations and more common duplications or triplications of a large genomic region that includes the α-synuclein (aSyn) gene (37). Clinically, PD is characterized by progressive neurodegeneration, manifesting in debilitating neurological symptoms such as increasing muscle rigidity, tremors, bradykinesia, and, in severe cases, near-total immobility (38). The motor symptoms arise from the degeneration of dopaminergic neurons in the substantia nigra, leading to a reduction in dopamine levels and the accumulation of intracytoplasmic Lewy bodies, which contain aSyn and ubiquitin (39). Dopamine is degraded by monoamine oxidase (MAO), a process that generates substantial quantities of hydrogen peroxide, which must be continuously neutralized by intracellular antioxidants to prevent oxidative damage (40).
3.2.1. Possible Mechanism Between Pesticide Exposure and Parkinson's Disease
3.2.2. Alpha-synuclein
Cymoxanil and metalaxyl, both pesticides, have been shown to induce aSyn aggregation, which disrupts normal cellular processes and ultimately leads to protein misfolding and aggregation (41). Chronic exposure to the pesticide dieldrin has been found to exacerbate aSyn preformed fibril-induced toxicity, particularly in male mice, thereby increasing their susceptibility to synucleinopathies and motor deficits (42). Additionally, certain pesticides, such as fluopyram, a mitochondrial complex II inhibitor, have been implicated in the pathogenesis of PD by inducing motor deficits and aSyn accumulation in the brain. This provides further evidence for the role of mitochondrial dysfunction in pesticide-induced PD (43).
3.2.3. ROS
Paraquat, a widely used herbicide, is strongly implicated in PD due to its ability to generate reactive oxygen species, diminish antioxidant defenses, and trigger apoptosis. It is recognized as a major environmental risk factor contributing to PD pathology (44).
3.2.4. Dysbiosis
Pesticides (organochlorines, glyphosate, pyrethroids, paraquat, and rotenone) are known to impact the microbiome-gut-brain axis, contributing to PD through dysbiosis and altered pesticide metabolism (45).
3.2.5. MicroRNAs
Pesticides also induce oxidative stress in neurons, and emerging research suggests that the deregulation of microRNAs (miRNAs) may be a critical link between pesticide exposure and PD. Specific miRNAs affected by pesticides (paraquat, OP, triazines, pyrazoles, organochlorines, conazoles, and rotenone) have been associated with the neurodegenerative processes characteristic of PD (46).
3.2.6. CTNNB1, NDUFS6, and CAV1
Key pesticides (benomyl, carbendazim, S-methyl-N-butylthiocarbamate, dichlorodiphenyltrichloroethane, dichlorodiphenyldichloroethylene, dieldrin, heptachlor, heptachlor epoxide, lindane, maneb, and rotenone) have been shown to bind with high affinity to dopamine neuron receptors, initiating signaling cascades that result in neurodegeneration. This study underscores the important role of certain genes, such as *CTNNB1* and *NDUFS6*, in pesticide-induced PD (47). Some biological mechanisms related to pesticides and PD are shown in Table 2.
| Pesticide | Mechanisms |
|---|---|
| Cymoxanil, metalaxyl, dieldrin and fluopyram | Alpha-synuclein aggregation |
| Paraquat | ROS |
| Organochlorines, glyphosate, pyrethroids, paraquat, and rotenone | Dysbiosis |
| Paraquat, OP, triazines, pyrazoles, organochlorines, conazoles, and rotenone | Oxidative stress and dysregulation of miRNAs |
| Benomyl, carbendazim, S-methyl-N-butylthiocarbamate, dichlorodiphenyltrichloroethane, dichlorodiphenyldichloroethylene, delidrin, heptachlor, heptachlor epoxide, lindane, maneb, and rotenone | CTNNB1 and NDUFS6 gene |
Pesticide and Parkinson's Disease Related Biological Mechanisms
4. Conclusions
With the increasing use of pesticides in various societies, the risk of exposure and subsequent acute and chronic effects has emerged. This mini-review highlighted the biological mechanisms of different pesticides on NDs. Pesticides are well-known environmental risk factors for the onset and progression of NDs (48, 49). Persistent or modest levels of exposure to pesticides such as PQ, MB, dieldrin, pyrethroids, and OP contribute to neurodegenerative illnesses such as AD and PD (14). Exposure to these pesticides increases tau phosphorylation through different mechanisms involving GSK-3β overexpression, increased Cdk5 activity, and decreased expression of PP2A, among other factors (22). Six review studies described in Table 2 reported increases in tau phosphorylation related to greater Cdk5 activity, changes in regulatory proteins MAPT and MAP-2, and increased oxidative stress, among other changes (22). Three studies (41-43) reported that pesticides (cymoxanil, metalaxyl, dieldrin, and fluopyram) could induce aSyn aggregation and accumulation, which ultimately contributes to PD. Dieldrin inhibits the ubiquitin-proteasome system (UPS), which clears misfolded or damaged proteins. This inhibition leads to the accumulation of aSyn aggregates in dopaminergic neurons (50). Additionally, pesticides like dieldrin, Paraquat, and others produce reactive oxygen species that damage macro/micro molecules and promote aggregation and misfolding of aSyn (44, 51). Another mediating mechanism involves inflammatory cytokines that activate microglial cells, which exacerbate aSyn in neurons (52). Considering the increasing use of pesticides in today’s societies, public health systems should control and reduce exposure to pesticides to prevent or address NDs. These disorders pose a significant burden to farming communities and, due to their complex pathology, their prevention presents a challenge. Our findings present valuable information to public health and medical experts on understanding NDs in vulnerable groups. Understanding the biological mechanisms behind pesticide exposure and NDs can improve the monitoring of the health status of farmers and nearby residents.