1. Context
Aptamers are small and specific DNA- or RNA-based single-stranded oligonucleotides that harbor the capability to fold into three-dimensional (3D) structures and bind to particular targets such as proteins, surface antigens, etc. They have demonstrated high therapeutic applicability due to their binding affinity towards malignancy-related antigens overexpressed in various types of hematological malignancies or solid cancers such as breast, lung, and colon cancer.
Cell surface biomarkers that are involved in various biological processes and pathways such as signal transduction, cell-cell interactions, cell adhesion, cell migration, and communication between the intra- and extracellular environments, are strongly important elements in various types of targeted cancer therapies including aptamer-based cancer therapy. A relation between the abnormal expression of cell surface biomarkers and tumorigenesis is often observed in various malignancies (1) and a high proportion of cancer-targeting drugs, such as therapeutic antibodies and small molecule inhibitors, have been developed that target these cell surface biomarkers (2). This makes these cell surface biomarkers interesting subjects for cancer treatment. Recently, many cell surface biomarker-targeting aptamers have been developed through the development of both protein- or cell-based SELEX technologies. A large number of these aptamers have been investigated in the diagnosis or treatment of hematological malignancies, as well as lung, liver, breast, ovarian, brain, colorectal, and pancreatic cancers (3-8).
Nucleolin is a protein engaged in various cellular functions including DNA metabolism and RNA regulatory processes such as transcription, translation, and ribosome assembly. It is predominantly located in the nucleolus alongside being present in the cytoplasm and the cell membrane (9). The dysregulation of nucleolin expression leads to the accumulation of nucleolin mRNAs and proteins and also its cell surface overexpression, in comparison with normal cells, is a hallmark of various cancers.
AS1411 is a nucleolin-targeting aptamer known as the most investigated aptamer in the field of cancer research. This fact highlights its strong potential and safety profile for clinical utilization. The first phase I study of AS1411 was initiated in September 2003 at the University of Louisville’s James Graham Brown Cancer Center (Louisville, KY) and it involved patients with a variety of advanced solid tumors who had progressive metastatic disease when they enrolled in the trial and who were incurable with the currently available treatment options. The results of this trial were promising for renal cell carcinoma and non-small cell lung cancer patients with objective responses, long-term disease stabilization, and no serious systemic toxicity. Moreover, in late 2007, the first phase II trial (NCT00512083) started at several institutions to treat patients with relapsed or refractory acute myeloid leukemia (R/R AML) (10). The trial completed in April 2009; however, no report of this trial has been posted on clinicaltrial.gov or publicly published yet. Furthermore, there is also another phase I clinical trial (NCT00881244) that has investigated the safety index and clinical efficacy of AS1411 in 30 participants with advanced solid tumors. Despite being completed in 2007, to this day, no reports have been published regarding the outcomes of the mentioned trial.
Various linking methods including covalent and non-covalent linking could be exploited for the attachment of different cargoes to AS1411 paving the way for linking a wide range of drug conjugates to this aptamer (10). Aptamer-drug conjugation (ApDC) is a straightforward and practical approach of conjugating aptamer sequences directly to therapeutic agents. This could lead to the preferential internalization of the conjugated drugs by tumor cells (rather than by healthy cells) which mediates enhanced therapeutic efficacy and reduces treatment-related side effects. Furthermore, nanoparticles (NPs) are exciting vehicles for aptamer-mediated delivery of different types of cargoes including drugs because they can increase both the drug half-life and the payload capacity of aptamer-based drug delivery systems. Alongside characteristics such as biocompatibility for clinical applications, drug loading capacity, and uniform size and shape for enhanced biodistribution, NPs have various additional physical and chemical characteristics based on the material used in their structure. Such characteristics could be exploited to improve biodistribution, stability, and targeting affinity of aptamers. For instance, metal materials offer extraordinary photothermal and magnetic performance, and copolymers and liposomes are nature-friendly and biodegradable. Consequently, using NPs provides an exceptional delivery system beneficial for the development of controlled-cargo release systems. In this review, we highlight how the nucleolin targeting aptamer, AS1411, can be utilized for improving the outcomes of cancer radio- and photothermal therapy and immunotherapy.
2. Novel AS1411-Equipped Delivery Nanosystems for Cancer Treatment
2.1. Delivery of Radiosensitizers
Radiation therapy (RT) is a localized treatment of cancer where high-energy radiation beams focus on the tumor. It is one of the most common and primary options available for cancer treatment benefiting more than half of the cancer patients (11). However, RT leads to damages to the healthy tissues while attacking the cancer cells. These unwanted damages still linger on as a factor limiting the broader success of this primary cancer treatment approach (12). To this date, different strategies have been proposed to overcome these limitations. Strategies such as inhibiting specific pathways that mediate radioresistance in tumor cells (such as NF-κB and MAPK) or attacking the tumor vasculature to augment tumor sensitivity to ionizing radiation (13). Here, we briefly discuss and highlight the advantages of nucleolin aptamer-mediated approaches for overcoming the unfavorable events of RT.
One strategy for the enhancement of RT efficacy and safety is the use of radiosensitizers. High atomic number materials, including gold nanoparticles (GNPs), are among the most popular radiosensitizers which have drawn major attention to themselves lately. Radiosensitizers improve radiation effects after the collision of radiation beams at the tumor site (14, 15). The small size of these nanoparticles makes them ideal candidates for reticuloendothelial system escape and sufficient accumulation in tumor cells (16, 17). They harbor a high level of renal clearance and are capable of minimizing side effects because of their appropriate excretion (16). Additionally, the decoration of GNPs with different types of cappings such as bovine serum albumin (BSA) leads to their enhanced internalization by tumor cells alongside rendering them more stable and biocompatible and giving them a uniform size (18-24). It is worth mentioning that smart targeting of these GNPs to tumor sites by conjugating them to antibodies or aptamers and their uptake and internalization by tumor cells but not by healthy ones is a factor of paramount importance. Therefore, smart targeting might come as a second strategy for improving the efficacy of RT. Conjugating BSA and gold nanoclusters (GNCs) to the AS1411 aptamer (Apt-GNCs) to obtain AS1411-functionalized BSA-GNCs can lead to a more specific tumor targeting strategy while increasing the internalization of gold nanoclusters by tumor cells (22-26).
Ghahremani et al. have investigated the radiosensitizing effects of the AS1411 aptamer-modified GNCs as tumor-specific radiosensitizers for improving the efficacy of megavoltage RT and enhancing the tumoral uptake of GNCs (25, 26). It has been demonstrated that the Apt-GNCs attached to nucleolin and were internalized by the target cancer cells. This mechanism led to the accumulation of significant numbers of Apt–GNCs in the tumor cells. Moreover, the interaction of radiation beams with intracellular Apt–GNCs increased the damage caused to cancer cells and improved radiation therapy efficacy (25, 26).
GNCs have been in the center of attention for their radiosensitizing properties (16, 17). This property of GNCs is due to the arrangement of the gold atoms. The atomic arrangement of GNC elevates the probability of radiation interaction with gold atoms. Moreover, their tiny size mediates their fast renal clearance making them materials with great levels of biocompatibility. This phenomenon can prevent the accumulation of these materials in unwanted and healthy organs and evade their trapping in the reticuloendothelial system (25, 26).
Other researchers have also investigated the utilization of the AS1411 aptamer for improving the outcomes of RT in glioma (27). They have reported that silver nanoparticles (AgNPs) functionalized with the AS1411 aptamer can selectively target C6 glioma cells with a great level of specificity (27). The results also indicated that the tumor cell internalization and the tumor mass penetrance of these AgNPs were satisfactory (27). Also, the comparison between the mentioned NPs with PEGylated AgNPs indicated that the AS1411-functionalized NPs exhibit superior radiosensitizing effects and are capable of inducing higher rates of apoptosis in tumor cells (27). However, it is known that PEGylation can enhance the solubility, stability, and circulation life of nanoparticles (28).
Additionally, in a recent study by Mehrnia et al., the radiosensitization effects of AS1411-functionalized gold nanoparticles have been investigated on various breast cancer cell lines (29). These researchers have demonstrated that functionalization of gold NPs with the AS1411 aptamer increased their cellular uptake in the MCF-7 and MDA-MB-231 cancer cell lines and the mammospheres of MCF-7 cells (29). This mechanism can enhance radiation-induced apoptosis in these cancer cells in vitro paving the way for more similar studies and preclinical studies regarding this effect.
Finally, these investigations propose that the AS1411 aptamer-GNCs conjugates improve the outcome of tumor radiotherapy by elevating the targeting power of RT and attenuating the unwanted damages to healthy tissues (25, 26).
2.2. Delivery of Photosensitizers
Photodynamic therapy (PTD) is a light-activated therapeutic modality utilized for the treatment of various malignancies. It is one of the most promising and non-invasive methods for treating malignant or premalignant tissues. In this method, radiation of a photoactive drug such as a photosensitizer with suitable wavelengths of light leads to the production of highly cytotoxic reactive oxygen species (ROS) causing fatal damages to cancerous cells (30, 31). However, the quantum yields and the systemic biodistribution of ROS are overshadowed by the hydrophobicity of the existing photosensitizers, which harbor limited solubility in aqueous solutions (30, 32). Moreover, pure photosensitizers also demonstrate limited selectivity towards cancer cells causing nonspecific photodamage to the cells of normal tissue (30). New strategies such as nanoparticle-based platforms for the delivery of photosensitizers have been contemplated to address these limitations. This new approach has several advantages such as maintaining the activity and stability of photosensitizers in aqueous solutions (30). Also, the nanoparticles themselves can be further functionalized with various types of targeting moieties for cancer-specific PDT (30). Furthermore, various porphyrin derivatives used as photosensitizers, such as N-methylmesoporphyrin IX (NMM), tend to have low fluorescence intensity aqueous solutions (30). Such porphyrin derivatives could achieve a remarkable fluorescence enhancement if linked to G-quadruplex DNA such as the AS1411 aptamer (30). They can also be applied as efficient photosensitizers for targeted cancer cell imaging and PDT (30).
As one of the first attempts in this regard, Shieh et al. conjugated six 5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphyrins (TMPyP4) to the AS1411 aptamer and demonstrated that this aptamer redirected the porphyrin derivatives to the MCF7 breast cancer cells overexpressing nucleolin (33). Additionally, they reported that the damages made to these cells after photodynamic therapy were significantly higher than the damages made to M10 normal epithelium cells (33). A fact that highlights the effects of the AS1411 aptamer selectively redirecting the photosensitizers towards the tumor cells (33).
Furthermore, other researchers have conjugated AS1411-functionalized fluorescent gold nanoparticles to the porphyrin derivative N-methylmesoporphyrin IX (NMM) (34). They have reported that the mentioned porphyrin derivative conjugated to AS1411-redirected NPs can be utilized as an efficient and specific photosensitizer for increasing the sensitivity of various tumor cell lines to PDT (34).
Another study by Zhu et al. has demonstrated that chemical photodynamic therapy nanosystems can be exploited as helpful and controlling synergistic tactics for fighting against brain tumors and various CNS-related brain diseases (35). These researchers have utilized ruthenium (II) polypyridyl complexes such as [Ru(bpy)2(tip)]2+ (RBT) in their AS1411-functionalized nanocarriers which can produce reactive oxygen species in target tumor cells under laser irradiation and induce apoptotic cell death improving the results of PDT in gliomas cells (35).
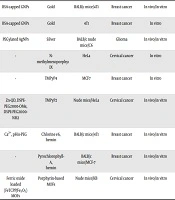
Some researchers have combined more intricate co-delivery systems which are composed of a therapeutic oligonucleotide such as a DNAzyme and a photosensitizer. In this regard, Jin and colleagues developed an upconversion nanoplatform composed of repetitive survivin DNAzyme and the AS1411 aptamer fabricated in a long single-stranded DNA (ssDNA) by rolling circle amplification (RCA) and eventually adsorbed on the upconversion nanoparticles (UCNPs) by electrostatic attraction (36). The enhanced photosensitizer (TMPyP4) and DNAzyme loading capacity of the upconversion nanoplatform are resulted from the multivalence of the ssDNA thus allowing for an enhanced PDT by DNAzyme-mediated gene silencing of survivin. The PDT effects of this platform were triggered by near-infrared (NIR) light after the internalization of the nanoplatforms into cancer cells resulting in the generation of ROS and their subsequent cytotoxic effects (36). Additionally, survivin DNAzyme acted to potentially enhance the efficiency of PDT by inhibiting the gene expression of survivin which conclusively demonstrated the efficacy of the upconversion photodynamic nanoplatform for combinatorial cancer therapy (36). Table 1 has brought together some nanosystems functionalized with the AS1411 aptamer for the delivery of radiosensitizers and photosensitizers.
| Type of Therapy/Description | Components | Radiosensitizer/Photosensitizer | Animal Models or/and Cell Line (s) | Investigated Cancer Type (s) | In Vivo/in Vitro | Reference |
|---|---|---|---|---|---|---|
| Radiation therapy | ||||||
| Via gold nanoparticles | BSA-capped GNPs | Gold | BALB/c mice/4T1 | Breast cancer | In vivo/In vitro | (25) |
| Via gold nanoparticles | BSA-capped GNPs | Gold | 4T1 | Breast cancer | In vitro | (26) |
| Via silver nanoparticles | PEGylated AgNPs | Silver | BALB/c nude mice/C6 | Glioma | In vivo/in vitro | (27) |
| Photodynamic therapy | ||||||
| Via fluorescent gold nanoparticles | - | N-methylmesoporphyrin IX | HeLa | Cervical cancer | In vitro | (34) |
| Via aptamer-photosensitizer complex | - | TMPyP4 | MCF7 | Breast cancer | In vitro | (33) |
| Via adenosine triphosphate (ATP)-activatable hybrid micellar nanoparticle | Zn-QD, DSPE-PEG2000-OMe, DSPE-PEG2000-NH2 | TMPyP2 | Nude mice/HeLa | Cervical cancer | In vivo/in vitro | (37) |
| Via nanoscale coordination polymers | Ca2+, pHis-PEG | Chlorine e6, hemin | BALB/c mice/4T1 | Breast cancer | In vivo/in vitro | (38) |
| Via aptamer-photosensitizer complex | - | Pyrochlorophyll-A, hemin | BALB/c mice/MCF-7 | Breast cancer | In vivo/in vitro | (39) |
| Via erythrocyte membrane-camouflaged nanorice | Ferric oxide loaded (FeTCPP/Fe2O3) MOFs | Porphyrin-based MOFs | Nude mice/KB | Cervical cancer | In vivo/in vitro | (40) |
Abbreviations: BSA, bovine serum albumin; GNPs, gold nanoparticles; AgNP, silver nanoparticles; TMPyP4, 5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphyrin; TMPyP, 5,10,15,20-Tetrakis(1-methyl-4-pyridinio)porphyrin tetra(p toluenesulfonate); QD, quantum dot; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000]; pHis, poly(L-histidine); MOF, metal-organic framework.
2.3. In Cancer Immunotherapy
Targeting nucleolin for antitumor purposes through the simultaneous administration of chemotherapeutic agents and immunostimulants has been known as a potent strategy capable of eliciting strong tumoricidal effects alongside immune responses (41). Here we describe the development of lipophilic-fused AS1411 aptamer-immunoadjuvant CpG-decorated (Apt-CpG-DSPE) high-density lipoproteins (HDL) for relay drug delivery and pronounced antitumor effects. The complete immune HDL nanodrug, hereafter referred to as imHDL/Apt-CpG-Dox also carried Dox successively intercalated into the consecutive base pairs of Apt-CpG (41).
Non-methylated cytosine-guanine oligodeoxynucleotides (CpG ODN) are Toll-like receptor 9 (TLR9) agonists which are capable of activating antigen-presenting cells (APCs) such as macrophages and dendritic cells as well as eliciting effective immune responses through the initiation of signaling pathways. Such signaling initiation results in the expression of pro-inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), interleukin-12 (IL-12), and various others (42-44). However, the antitumor application of CpG ODN is intertwined with various obstacles such as their in vivo instability, nonspecific biodistribution, and unfavorable pharmacokinetics (41, 45, 46). Despite the development of various nanotechnology-based delivery systems significantly improving the delivery profiles of CpG ODN, there are remaining concerns about the use of these synthetic nanomaterials (41, 45, 46). Therefore, natural material-based nanoscale delivery systems such as those based on human HDL are more desired because of their high molecular-level structural adaptability, biocompatibility profile, and excellent delivery functionality (47, 48).
Due to the rapid proliferation of malignant cells, they grow a substantial need for cholesterol therefore they up-regulate the expression of HDL-related surface receptors such as scavenger receptor class B type I (SR-BI) to trap HDLs from the systemic circulation (41). The monolayered HDLs used in Han and colleagues’ study (41) are also surface-anchored with the lipophilic macromolecule apolipoprotein A-I (apoA-I) to further stabilize their nanostructure for systemic circulation and tumor site-specific drug translocation (49, 50).
The drug delivery platform discussed here is composed of two sequential modules. As described by Han et al., after intravenous (IV) injection of immune lipoprotein imHDL/Apt-CpG-Dox and their migration to the tumor tissue site, they underwent extracellular structural collapse upon the recognition of surface SR-BI (sequential module I) (41). After extracellular dissociation of the HDL nanostructures, the endocytosis of the dissociated Apt-CpG-Dox into tumor cells is mediated by the interaction of the AS1411 aptamer with nucleolin (sequential module II) (41). After internalization, Apt-CpG-Dox was translocated to the nucleus via AS1411-nucleolin interaction where Dox release was mediated by the pH level decline in endo-lysosome resulting in malignant cell apoptosis alongside in situ tumor-associated antigens release (41). Moreover, the dying tumor cells unleashed the CpG motifs which then were internalized into the TLR9-rich infiltrated APCs leading to their activation and subsequent promotion of proinflammatory cytokine release resulting in potentiated host antitumor immunity (41). In conclusion, this HDL biomimetic-based relay drug delivery system exhibits therapeutic advantages over its monotherapy counterparts by generating pronounced antitumor effects against malignant tumors (41).
3. Summary and Perspectives
New approaches efficient in ameliorating the side effects of common cancer treatment modalities are of great importance since the side effects can be success-limiting and life-threatening based on the disease condition of the patients (51, 52). The clinical application of radiation therapy and photodynamic therapy are very common as primary options for the treatment of various types of cancer. Radiotherapy-related side effects, the slow recovery process of the patients under radiotherapy, the development of radiation-resistance mechanisms by cancer cells, and the low efficiency of photodynamic therapy are all among important factors that have limited the boundaries of success for these approaches. The non-specific cell targeting manner of these approaches also leads to irreversible damages to normal cells alongside causing cancer cell fatality.
Additionally, using immunotherapy for fighting against cancer cells has been investigated in various bodies of research (51, 52). However, cancer immunotherapy itself can cause mild to severe levels of toxicity (51, 52). To address these unfavorable downsides, cancer immunotherapy can be combined with other novel treatment approaches to create more successful results (51, 52). In this regard, nanotechnology-based delivery systems are irreplaceable candidates since they can easily be exploited for achieving the mentioned aim. However, nanotechnology-based delivery systems suffer from drawbacks such as not having a selective redirection system to avoid unwanted damages by discriminating between normal and cancerous tissues. Since these drawbacks have overshadowed the broad applicability of nanotechnology-based delivery systems, they require meticulous optimizations for a precise tumor cell-specific redirection. Even though there have been many advances in the field of nanotechnology-based delivery vehicles for targeted cancer therapy, the main success-creating factor in this field is steering these vehicles only towards cancer cells to achieve the lowest level of off-target toxicity and the highest level of efficiency.
Undoubtedly, the AS1411 aptamer might be considered a great solution for the mentioned problem. This aptamer has also been utilized in the delivery of different types of photo-sensitizers to target cancer cells rendering them susceptible to photodynamic therapy. It has also been exploited in specific delivery of radiosensitizers to target cells leading to efficient radiation therapy with less unwanted damages to healthy cells. Cancer immunotherapy is also another field benefiting from the AS1411 aptamer. The utilization of this aptamer in cancer immunotherapy results in the generation of pronounced host antitumor immunity effects. Soon, the AS1411 aptamer can bring more surprising results to the table of common cancer treatment approaches and it can improve the results of these therapies more than it already has. However, it is worth mentioning that various studies discussed here or other similar studies regarding the topic of this article need to be more investigated meticulously in preclinical models to be evaluated more accurately in terms of applicability and safety. It is still too soon to state that these platforms can mediate promising results in clinical trials since the available outcomes of the herein discussed studies lack certain in-depth assessments. For instance, some of these platforms have only been assessed in vitro or a large proportion of them have been investigated only against cancer cell lines. In this regard, their assessments in preclinical animal models of human tumors might result in high-grade toxicities towards healthy tissues as a result of their poor tumor-specific uptake in some cases. Furthermore, some of these strategies might not behave in preclinical animal models as they have in vitro. This phenomenon could be attributed to the fact that in vitro studies do not completely mirror the complexity of animal models. In a similar scenario, even if successful outcomes are achieved in the preclinical investigations, the evaluation of clinical trials might present different findings since animal models do not bear the same level of complexity as human beings. However, for now, the mentioned facts make it impossible for these platforms to meet the minimum level of validity for translational purposes.