1. Context
More than three decades after the first attempt to use monoclonal antibodies (mAbs) for cancer therapy, today, mAb-based cancer therapies are rapidly developing (1-4). Currently, numerous mAbs have been approved by the US Food and Drug Administration (FDA) for medical use in various fields (5). These commercially available mAbs exert their functional effects through binding to cell surface receptors or targeting ligands that are soluble (6, 7). Upon such interactions, a unique downstream signaling pathway will be triggered, which usually disrupts a molecular signaling pathway, consequently leading to the blockage of the proliferation signaling of malignant tumor cells (8, 9). Therefore, tumor cells can be eliminated in a very selective manner in comparison with the commonly available cancer treatment methods such as chemotherapy. Moreover, antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are also other mechanisms by which mAbs mediate the elimination of their target cells (10). In these mechanisms, mAbs can activate the complement or engage the effector cells of the immune systems to destroy and eliminate tumor cells towards whose cell surface antigens the mAbs have binding affinity (10). mAbs have also been utilized for redirection purposes. In this regard, they have been used for the surface decoration of nanoparticles, which can be loaded with various types of cargoes, including cytotoxic chemotherapeutic drugs, toxins, nucleotides with therapeutic value, photosensitizers, and radiosensitizers (11-13). Despite the advantages of these platforms, there are several hindrances as well, such as the large size of mAbs (even without linking them to nanoparticles), which overshadows their tumor site penetration and trafficking capability (14-16).
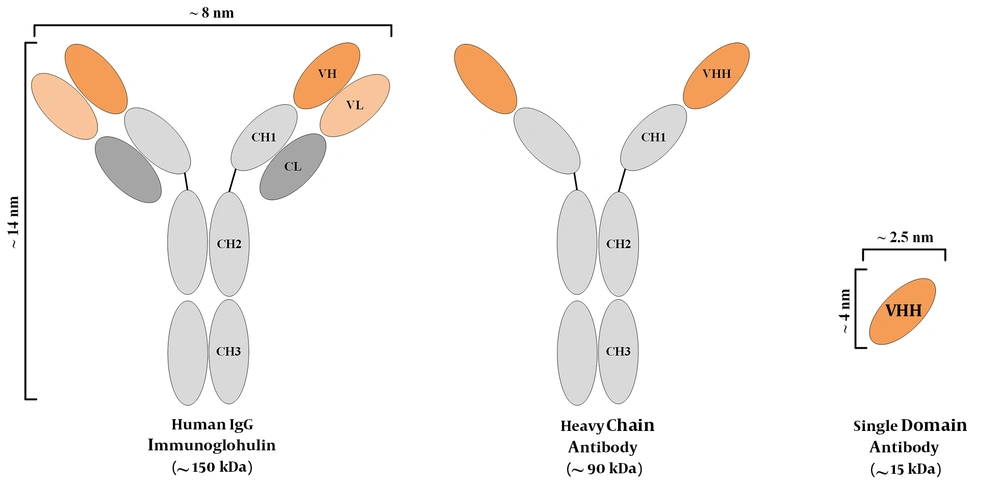
Typically, in mammals, plasma cells, which are the immunoglobulin (Ig)-secreting type of B cells, secrete antibodies that are composed of two heavy chains and two light chains. Such antibodies are known as full-length antibodies. Each chain of these full-length antibodies harbors variable and constant regions. However, there are natural or synthetic types of antibodies that are made of only a part of full-length antibodies (17). These smaller structures include antigen-binding fragment (Fab), variable fragment (Fv), or single-chain variable fragment (scFv). These small antibody fragments offer great advantages over the conventional full-sized ones; however, they also suffer from several drawbacks. In comparison to the full-length antibodies, Fabs, Fvs, and scFvs tend to have diminished stability, generally lower affinity, and production difficulties (18).
The discovery of an additional IgG isotype, made of only a homodimer of heavy chains, has been known as a revolution in the field of antibody investigations (19, 20). Since then, heavy-chain antibodies (HCAbs) have been recognized as potential targeting tools in various developmental and clinical investigations. The outstanding part is that even though these antibodies do not harbor light chains, they are completely functional and they tend to have the same level of affinity to their specific antigen, as compared with that of conventional murine or human antibodies. These heavy-chain antibodies are mostly attained from the Camelidae family members, including camels and alpacas. It is believed that the simple process of immunizing these animals is the main reason behind this choice, considering the fact that other animals such as sharks also harbor HCAbs (19). Ever since the discovery of HCAbs, researchers all over the world have exploited the variable domain of the heavy chain (also known as a VHH or a nanobody®) of such HCAbs for therapeutic and diagnostic purposes, and they have reported encouraging outcomes (4, 21, 22). Figure 1 is a simplified representation of a conventional antibody, an HCAb, and a VHH. Nanobodies also tend to have other favorable characteristics such as their high solubility and stability rates (23, 24), their simple production process in bacteria (allowing for large-scale productions) (25), their outstanding tissue trafficking capability (resulting from their small size) (26), and their rapid clearance from the blood circulation (27). However, the rapid elimination of antibodies from the circulation tends to be an unfavorable factor in therapeutic applications, where long-term persistence is required for efficient responses. Moreover, due to their origination from camelid species, they tend to be immunogenic in humans. In this regard, humanization may be helpful for minimizing the immunogenicity of nanobodies (28). So far, various nanobodies have been developed against numerous targets involved in different oncological and immunological indications, some of which have been summarized in Table 1. In this review, we briefly discuss the use of VHHs in various fields, including direct cytotoxic drug conjugation, signaling blockade purposes, VHH-facilitated redirection of delivery systems, and cancer immunotherapy.
| Nanobody Designation | Target Antigen | Involved Indication | Animal Source | Reference |
|---|---|---|---|---|
| H11 | CTLA-4 | Various oncological indications | alpaca | (29) |
| Nb_7, Nb_21, and Nb_22 | CD33 | AML | Llama glama | (30) |
| 11A4 | HER2 | Breast cancer | Llama glama | (31) |
| SRB-37 and SRB-85 | CD19 | Hematologic malignancies | Camelus bactrianus | (32) |
| RR-B7 and RR-D40 | MUC1 | Advanced solid tumors | Camelus dromedarius and Camelus bactrianus | (33, 34) |
| NB5 | CXCR7 | Neck cancer | Llama glama | (35) |
| Anti-TNF-VHH | TNFα | Solid tumors | - | (36) |
| VHH4 | MHC-II | GvHD | Vicugna pacos | (37) |
| C3, E2 | PD-L1 | Advanced solid tumors | Camelus dromedarius | (38) |
| K2 | PD-L1 | Advanced solid tumors | alpaca | (39) |
| cAb-CEA5 | CEA | Breast, colorectal, ovarian, pancreatic, and cancer | Camelus dromedarius | (40) |
| JJB-B2 | CEA | Breast, colorectal, ovarian, pancreatic, and cancer | Vicugna pacos | (41) |
| HuNb1-IgG4 | CD47 | NHL, colorectal cancer, ovarian cancer, and AML | Camelus bactrianus | (42) |
| C3 | PSMA | Prostate cancer | Camelus bactrianus | (43) |
| N14, C24, C9 | PSMA | Prostate cancer | Camelus dromedarius | (44) |
| MU738, JK2, WF211, MU523, and MU1067 | CD38 | Multiple myeloma | Llama glama | (45) |
| 127D1 and 163E3 | CXCR2 | Solid tumors and immunological indications | Llama glama | (46) |
| VHH6 | CD7 | Leukemias | Llama glama | (47) |
| V36, 81, 51, and B10 | CD11b | Immunological indications | Llama glama | (48) |
| K24 | CAIX | Breast cancer | Camelus dromedarius | (49) |
Abbreviations: AML, acute myeloid leukemia; NHL, non-Hodgkin’s lymphoma; GvHD, graft versus host disease.
2. Nanobodies in Therapy
2.1. Nanobodies Application in Infectious Diseases
The neutralizing effects of nanobodies have been promising in various studies paving the way for their investigation in various kinds of virus- or bacteria-induced conditions. In this regard, researchers have investigated the protective effects of llama-derived hemagglutinin-targeting nanobodies in preclinical models of influenza A virus subtype H5N1 (50). They demonstrated that the intranasal administration of these nanobodies successfully suppresses influenza A virus replication (50). They also added that using bivalent nanobodies may lead to a more efficient virus replication suppression than using monovalent nanobodies, in this case (50). Moreover, Hussack et al. investigated the neutralization of Clostridium difficile toxin A using nanobodies targeting its cell receptor-binding domain (51). To date, Clostridium difficile has been a significant challenge for healthcare professionals in hospitals (51). The authors of the mentioned study concluded that these nanobodies show effective toxin neutralization alongside exhibiting a satisfactory level of stability (51).
Researchers have reported that high oral doses of the recombinant nanobody clone K609, which is directed against Escherichia coli F4 fimbriae, and decrease diarrhea induced by E. coli in piglets (52). The same research group further investigated the stability of a more stable clone of these nanobodies named K922 (52). They demonstrated that K922 exhibits significant binding affinity to F4 fimbriae, which is higher than that of K609 (52). They also added that K922 inhibits fimbrial adhesion at lower doses in comparison with that of K609 (52). Ultimately, they concluded that K922 may be a favorable choice for the prevention of diarrhea in piglets (52).
Malassezia furfur is a yeast species known as the reason for various dermatological diseases (including dandruff) triggered by fungal infections (53). Dolk et al. have demonstrated that llama-originated nanobodies isolated using the phage display method can effectively inhibit the growth of organisms associated with dandruff (53). This study indicated that detergent-resistant species of nanobodies targeting the surface protein of Malassezia furfur can be generated for application in anti-dandruff shampoos (53).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a recently emerged virus that causes coronavirus disease 2019 (COVID-19). SARS-CoV-2 viral particles harbor an envelope protein called spike (S) which interacts with the cell surface receptors (known as ACE-2) of their host cells mediating virus entry into cells (54). Considering the vital role of the spike protein in the functionality of these viruses, it has become a popular target for blocking the entry of the virus into the host cells and preventing the replication of the virus (54). In 2020, Wrapp et al. isolated VHHs from a llama immunized with coronavirus spikes (54). They demonstrated that these nanobodies were capable of binding to the ribosome binding domain (RBD) of the spike protein and neutralizing coronaviruses such as SARS-CoV-2 (54).
2.2. Nanobody-Drug Conjugates
Nanobody-drug conjugation, powered by the selective targeting ability of nanobodies, is another platform that has shown promising results in targeted cancer therapy investigations. The main advantage of this approach is that it is capable of alleviating the off-target side effects of the unconjugated therapeutic agents. Nanobodies have attracted much more attention towards themselves since they tend to have advantages over full-length antibodies. The main advantage of nanobody-drug conjugates in comparison with full-length antibody-drug conjugates is that they can penetrate the tumor sites more easily due to their small size.
Wu et al. conjugated Pt(IV) prodrugs to anti-EGFR nanobodies to selectively deliver them to tumor cells (55). They showed that this platform is capable of inducing antitumor effects both in vitro and in vivo (55). They added that clustered nanobody-drug conjugates exhibit superior antitumor effects in vivo as compared to those of monomeric nanobody-drug conjugates (55). Such pronounced antitumor effects could be a result of the prolonged circulation time and lower kidney clearance rate of clustered nanobody-drug conjugates in comparison with monomeric nanobody-drug conjugates, which are smaller in size.
Our investigational team has conjugated the PE38 toxin (a truncated form of Pseudomonas exotoxin A) to nanobodies and has shown that this nanobody-toxin conjugate can exhibit selective tumor cell targeting ability and cytotoxicity towards tumor cells that express the target antigen (data under publication). Moreover, our experience demonstrates that the nanobody-toxin conjugate platform might serve as a flexible and potential treatment modality against various hematologic malignancies and solid tumors.
2.3. Their Application in Checkpoint Blockade Therapies
Checkpoint blockade therapy is a type of immunotherapy that uses a wide range of agents, from chemical drugs to mAbs, to block the signaling pathway of immune checkpoints or other critical regulators of the immune system (56-60). PD-1 and CTLA-4 are two well-known T cell exhaustion markers whose blocking using mAbs has been effective in creating potent antitumor responses in various types of cancers (56-60).
KN035 is a PD-L1-targeting nanobody capable of mediating robust T cell antitumor activity and suppressing tumor proliferation in preclinical in vivo models (61). Furthermore, a CTLA-4-targeting nanobody named H11 has shown promising results in suppressing tumor growth in preclinical mouse models of melanoma (29).
Ma et al. have generated multivalent bispecific antibody (BsAb) co-targeting PD-L1 and TIGIT (also called T cell immunoreceptor with Ig and ITIM domains) (62). TIGIT is a recently known immune checkpoint molecule that has mediated encouraging results in immune checkpoint blockade therapies (62). The BsAbs are made of a tetravalent anti-PD-L1 Fc-fusion nanobody and a tetravalent anti-TIGIT nanobody (62). The results of this study indicated that the anti-PD-L1 nanobodies harbor significant levels of specificity and affinity against PD-L1, and they can improve T cell functionality and antitumor activity both in vitro and in vivo (62). The researchers also added that similar results were also obtained for the anti-TIGIT nanobodies as these nanobodies had significant levels of specificity and affinity towards TIGIT (62). Furthermore, Ma et al. also reported that the BsAb construct was very effective in disrupting both the PD-1/PD-L1 and the TIGIT/CD155 axis in vitro (62).
2.4. Nanobody-Facilitated Redirection of Delivery Systems
As delivery systems are becoming increasingly popular, various redirection methods have been investigated for the targeting of these platforms to the tissues and cells of interest (63, 64). Delivery platforms, including liposomes, nanoparticles, micelles, etc. have been investigated for the delivery of various cargoes such as therapeutic drugs (e.g., doxorubicin, paclitaxel, and cisplatin), miRNAs, siRNAs, toxins, etc., since they can mediate the controlled release of their cargoes in the intended tumor sites they are targeted towards (63, 64). These delivery platforms have differences in their mechanism of action and structures, but the common feature among them is the presence of a targeting moiety in their constructs (63, 64). The targeting moieties of these delivery platforms are usually decorated on their surface, allowing their easy interaction with the target tumor cell surface antigens (63, 64). So far, various targeting moieties have been utilized for the redirection of these delivery platforms, all of which have their advantages and disadvantages (63, 64). Besides mAbs, other targeting moieties such as aptamers have also been investigated in this regard (63, 64). However, in comparison to aptamers, mAbs have various advantages. The process of generating a specific mAb against a cell surface of interest is more feasible than that of aptamers. Furthermore, the affinity and specificity of mAb tends to be more selective towards cell surface antigens of interest expressed by tumor cells in comparison with those of aptamers.
As a contribution to this topic, researchers have generated extracellular vesicles surface-decorated with anti-EGFR nanobodies and have demonstrated that these vesicles are specifically redirected towards EGFR-expressing tumor cells (65). Moreover, Liu et al. investigated the applicability of EGFR nanobody surface-decorated polymeric micelles loaded with photosensitizers (66). These researchers loaded the polymeric micelles with the photosensitizer temoporfin (mTHPC). This platform proved efficient in redirecting polymeric micelles towards EGRF-positive cancer cells in vivo (66). The researchers also added that these polymeric micelles tend to have enhanced circulation and diminished the level of clearance as compared with those of naked mTHPC (66). Other researchers have developed tumor-targeting nanobullets which are made of liposomes surface-decorated with anti-EGFR nanobodies (67). These liposomes were loaded with the anti-IGF-1R kinase inhibitor AG538 (67). The researchers of this study indicated that the mentioned nanobullets were capable of inducing robust tumor cell growth suppression in a very efficient and selective manner (67).
3. Conclusions
The favorable characteristics of nanobodies make them exclusive and irreplaceable therapeutics for various types of treatments. For instance, caplacizumab (also known as Cablivi) achieved FDA approval for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura (aTTP) in 2019 (68). Moreover, as discussed throughout this article, the big size of the conventional full-length antibodies creates limitations in tissue penetration itself. The mentioned size limitation leads to more hurdles when these antibodies are bound to delivery vehicles such as nanoparticles for the aim of redirection. In this regard, nanobodies can be alternatives to full-length antibodies for the redirection of delivery vehicles such as nanoparticles with great levels of specificity and affinity. In the case of nanobody-drug conjugates, the same story applies. Therefore, utilizing nanobodies as the targeting moiety of these nanoparticles or nanobody-drug conjugates enables creating more effective platforms. Furthermore, nanobodies have also proven efficient in checkpoint blockade therapies.
Herein, we discussed some of the studies investigating the suitability of nanobodies for fighting against infectious diseases. However, there is still room for improving the functionality of nanobodies. Approaches such as bivalent nanobodies can be used to enhance the affinity of monovalent nanobodies (69). Moreover, there are methods used for elevating the in vivo half-life of nanobodies (22, 69). The fusion of nanobodies to human serum albumin is a great example used for elevating the in vivo half-life of nanobodies (22). Bivalent nanobodies also tend to have superior half-life in the circulation system in comparison with that of monovalent nanobodies (70). However, such innovative nanobody engineering methods require careful in vivo assessments for determining their safety and applicability in various types of therapies. Moreover, nanobodies developed by such engineering techniques should also be meticulously examined in terms of their immunogenicity, stability, and affinity. It is also speculated that these modifications may have negative impacts on various characteristics of nanobodies, such as their affinity, tissue penetration, stability, and solubility. Once the outcomes of in-depth assessments or clinical trials come out, the ambiguities remaining in this field can be cleared out. Therefore, it can be safely concluded that nanobodies might be beneficial in achieving more efficient treatment modalities.