1. Background
The invasion of placental cytotrophoblast cells to the maternal spiral arterioles leads to vascular remodeling of endothelial cells. The major physiological role of the placenta is vascular network development for nutrition and other exchanges between fetal and maternal blood circulation. The placenta can produce angiogenic factors, such as vascular endothelial growth factor A (VEGF-A) and placental growth factor (PLGF) (1). Some studies have established the essential role of angiogenic and antiangiogenic factors that act locally via their receptors, thereby controlling the vascular remodeling. Some types of high-affinity VEGF receptors (e.g., VEGFR-1 and VEGFR-2) and soluble fms-like tyrosine kinase-1 (sFlt-1) have an important role in the modulation of vascular remodeling (2). The balanced level of endothelial cells plays an important role in vascular remodeling in pregnancy. Their imbalances are associated with endothelial insufficiency (3). In vivo studies show that antiangiogenic factors, such as sFlt-1, and a soluble form of the transforming growth factor β receptor or soluble endoglin (sEng) can induce endothelial dysfunction and maternal syndrome of preeclampsia (4).
The sFlt-1, a potent antiangiogenic factor produced by messenger ribonucleic acid (mRNA) alternative splicing of encoding cell-membrane mFlt-1 (membrane-bound Flt-1), can trap VEGF ligand. The sFlt-1 is expressed in numerous tissues and vascular endothelial cells, such as placental trophoblasts and hypoxia-stressed smooth muscle cells (5). Particularly, placental trophoblasts express several folds more sFlt-1 than Flt-1 mRNA and protein levels (4).
Recently, a sFlt-1 variant, sFlt-1 e15a, as a potential biomarker was measured by a newly developed enzyme-linked immunosorbent assay (ELISA) in women with fetal growth restriction and preeclampsia (6). The sFlt-1, PLGF, and sEng are assessed as the diagnostic biomarkers of preeclampsia extensively (7, 8). Currently, the sFlt-1/PLGF ratio has a diagnostic value for placental dysfunction-related disorders, especially in the case of more severe and/or early forms of preeclampsia (9).
Investigations have confirmed the important role of local oxygen availability in human trophoblast cell differentiation (10, 11). In the cells, oxygen can regulate gene expression by inducing the hypoxia-inducible factor (HIF) and effect on hypoxia-responsive proteins in the promoter of different genes (12, 13). The expression of numerous proteins, such as PLGF, VEGF, its receptors, and related proteins (i.e., VEGF and sFlt-1), is induced under low-oxygen conditions via the HIF pathway (11). According to Korkes et al., in preeclampsia placentas, the hypoxia-inducible factor-1α (HIF-1α) protein level is about two folds higher than the normal placenta. They also reported positive feedback between miR-210 and HIF-1α in these patients (11).
The hypoxia-mediated alteration of the VEGF family is involved in the pathogenesis of placenta-related diseases, especially preeclampsia as angiogenic imbalance (13) and serious complication that affects 5 - 8% of all pregnancies. The sFlt-1 and PLGF can be used to diagnose and predict the adverse outcomes of the disease (14). Syncytiotrophoblast cell stress leads to biochemical changes in the levels of sFlt-1 and PLGF during the last 8-10 weeks of pregnancy (15).
Hypoxia is a major factor in releasing sFlt-1. The upregulation of sFlt-1 can be related to oxidative stress created after hypoxia in placental trophoblast cells (13). Increased ER stress proteins, such as glucose-regulated protein-78, eukaryotic initiation factor-2α, X-box binding protein 1, activating transcription factor 6, and C/EBP-homologous protein, in trophoblast cells with a high level of sFlt-1 indicate that they are related to oxidative stress and might cause endoplasmic reticulum stress (16).
The sFlt-1 as an inflammatory marker might also contribute to hypoxia and stabilized HIF-1. Tumor necrosis factor α can provoke sFlt-1 release from cultured placental explants (17, 18). Oxidative stress mediated by reactive oxygen species can increase sFlt-1 releasing via nuclear factor kappa B (NF-κB) at the same or greater levels, compared to hypoxia (19).
N-3 long-chain polyunsaturated fatty acids (n-3 LCPUFAs) are the essential ingredients of membrane for maintaining cell integrity. Additionally, they are important for gene expression as intracellular mediators. Fatty acid composition changes can modify the fluidity and thickness of the membrane, create specific interactions with active membrane proteins, deform lipid rafts, inhibit transcription factor NF-κB for decreasing inflammatory gene expression, and overexpress transcription factor peroxisome proliferator-activated receptor-gamma as an anti-inflammation and alters eicosanoids balance (20).
2. Objectives
The investigation of eicosapentaenoic acid (EPA) effect on sFlt-1 mRNA expression and sFlt-1 secretion in the JEG-3 cell culture under hypoxia-like conditions.
3. Methods
JEG-3 choriocarcinoma cells (trophoblast cell model) were purchased from Pasteur Institute of Iran. The cell culture essential ingredients and fetal bovine serum (FBS) were purchased from Invitrogen Corporation, UK. Dimethyloxalylglycine (DMOG), dimethyl sulfoxide (DMSO), and EPA were purchased from Sigma-Aldrich, USA. Cell proliferation and viability kit [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)] were obtained from Roche Applied Science, Germany. SYBR Green PCR Master Mix and RNA extraction kit were purchased from Qiagen, USA. Other materials were of analytical grade.
3.1. Dose-Dependent and Time-Dependent Cell Toxicity
Mitochondrial viability was evaluated using the MTT method (16). The effects of DMSO (0.1%), EPA (12.5 - 100 µm), and DMOG (50 - 300 µM) on JEG3 cells viability were evaluated with the cells cultivated in microplates at a final volume of 100 µL culture media per well. The cells were incubated with DMSO, DMOG, and EPA for 12, 24, and 48 hours. Afterward, 10 µL MTT reagent was added and incubated for 4 hours. After the addition of solubilizing solution (100 µL), it was incubated at 37°C to dissolve formazan crystals overnight. The results were recorded using the ELISA reader (Stat Fax 4200) at the wavelength of 650 nm.
3.2. Cell Culture and Treatments
Trophoblast tumor cell lines (JEG-3 cells) were cultured in minimum essential medium eagle with 10% FBS and maintained at 37°C. For studying the effects of EPA on sFlt-1 level, all treatments were carried out under serum-free conditions. The cells were treated with EPA (50 µM), DMOG (100 µM), and DMOG/EPA (in triplicate) for 24 hours. The DMOG exerts 2% hypoxia in the culture (10). Controls without EPA or DMOG were prepared at the same conditions.
3.3. RNA Extraction and Quantitative Real-Time PCR Analysis
The RNA of the cells was extracted by the RNeasy kit (Qiagen, USA), and its concentrations were estimated by the measurement of the optical density at 260 nm; then, QuantiTect Reverse Transcription Kit (Qiagen, USA) was used. QuantiTect SYBR Green PCR Kit (Qiagen, USA) for real-time polymerase chain reaction (PCR) was performed under the conditions of 95°C for 30 seconds, 56.2°C for 30 seconds, and 72°C for 20 seconds (about 40 cycles). Standards and complementary deoxyribonucleic acid samples were amplified using specific primers for sFlt-1 (forward, 5/- CAGCGCATGGCAATAATAGA-3/; reverse, 5/-TTTCTTCCCACAGTCCCAAC-3/; 121-bp product). Glyceraldehyde-3-phosphate dehydrogenase as a housekeeping gene for internal control was used for normalization.
3.4. Western Blot Analysis
The Western blot analysis of SFlt-1 and HIF-1α was performed by loading 5 0µg (20 µL) JEG3 cell protein lysate on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferring to nitrocellulose membranes. Nitrocellulose membranes were located in 5% bovine serum albumin and washed three times to avoid nonspecific bonds. The membranes were incubated in 10 mL primary antibodies (Human Flt-1 monoclonal antibody mouse IgG R&D # MAB321-100 and Human HIF-1α monoclonal antibody mouse IgG R&D # MAB1536-SP) in appropriate dilution with gentle agitation overnight at 4°C. Then, they were washed and located in horseradish peroxidase-conjugated secondary antibody (goat anti-mouse IgG polyclonal antibody Thermo scientific # 31160) at room temperature for 1 hour. Subsequently, solutions of 3, 3’-diaminobenzidine and hydrogen peroxide were added on nitrocellulose membranes, and dark brown blots were observed (21).
3.5. Statistical Analysis
The results are presented as mean±standard deviation. Differences between the groups were determined by one-way analysis of variance, followed by Tukey’s multiple comparisons posthoc test. The level of significance was set at less than 0.05. Data analysis was performed using SPSS software version 17.0.
4. Results
4.1. Cell Viability Using MTT Assay
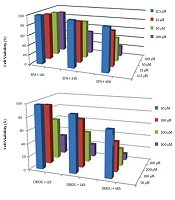
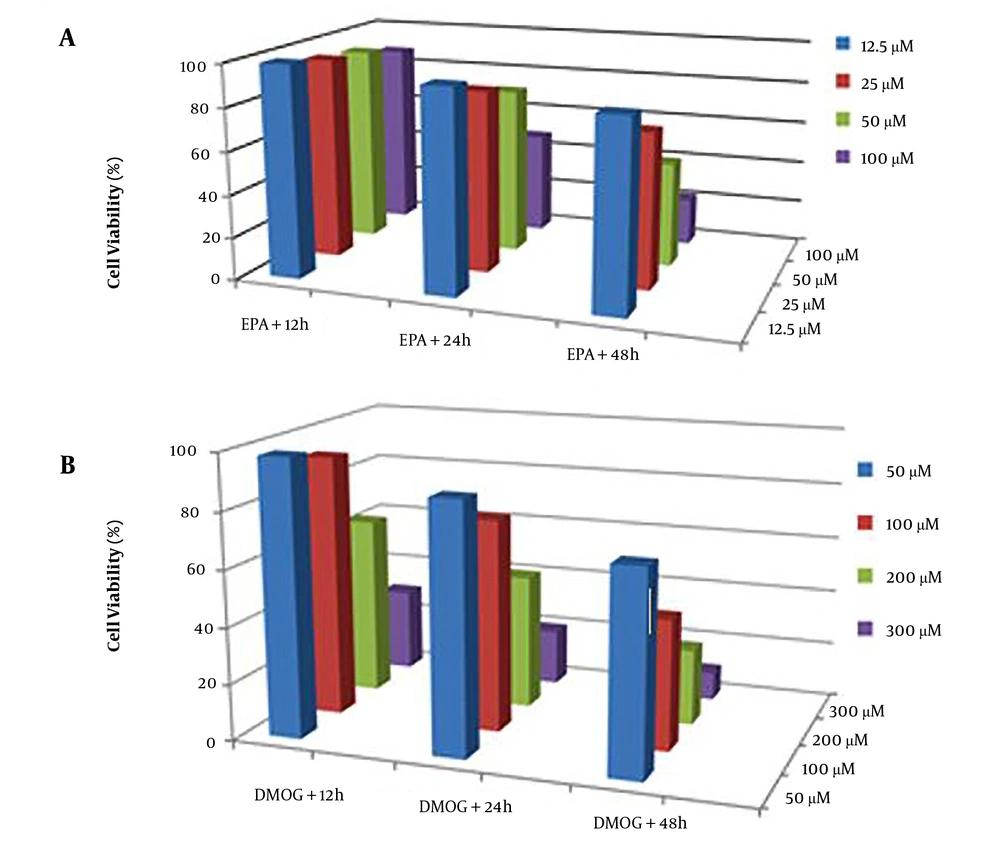
The cells were grown in a culture medium after exposure to the considered compounds under the same conditions and were harvested for subsequent experiments. For the determination of the best nontoxic condition, the toxicity of different concentrations of EPA (Figure 1A) and DMOG (Figure 1B) on JEG3 cells was investigated at various time points. The results indicated that the experimental concentrations of 100 µM DMOG as a hypoxia-like condition (P < 0.05) and 50 µM EPA did not induce cell death at 24 hours (22). Therefore, these concentrations were used in subsequent experiments.
4.2. Western Blot Analysis
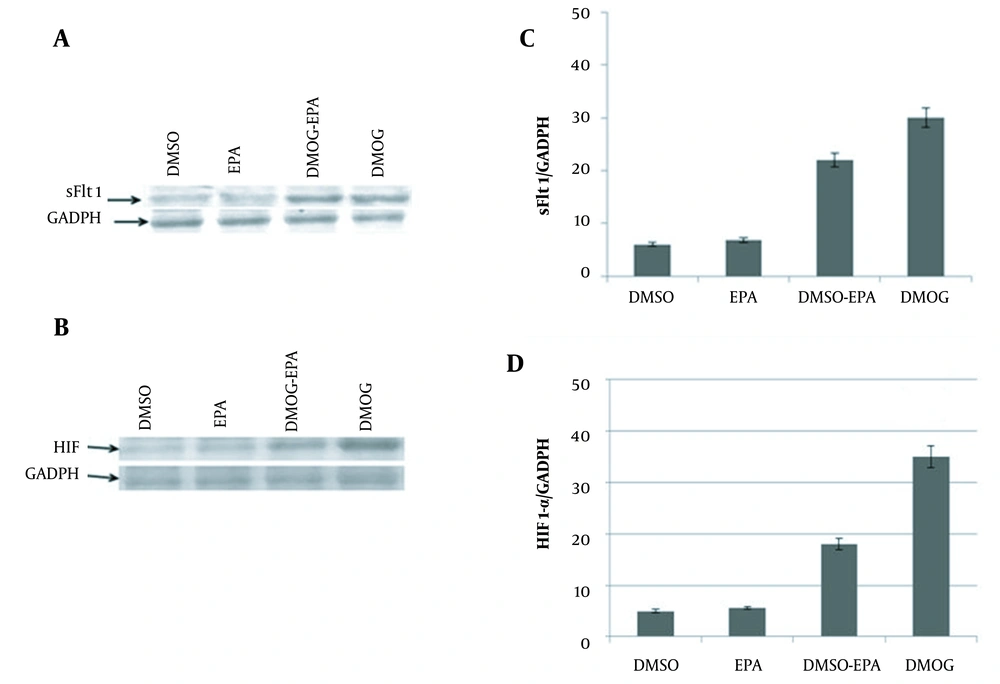
The immunoblotting assay showed that in the hypoxic condition (induced by DMOG), the expression of sFlt-1 (Figure 2A) and HIF1-α (Figure 2B) were significantly increased. Western blot analysis demonstrated the decreased secretion of sFlt-1 (Figure 2C) and HIF-1α (Figure 2D) in a hypoxic condition treated with EPA (DMOG + EPA); however, EPA could not change their expression levels.
4.3. Quantitative Real-Time PCR Analysis
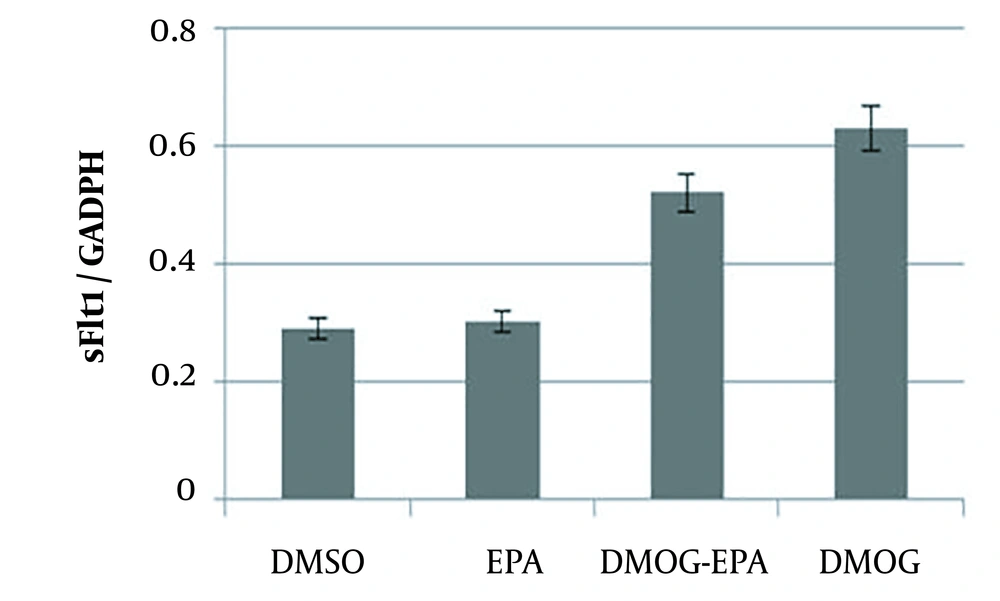
Real-time PCR analysis showed that sFlt-1 level did not change with EPA (P = 0.261); nevertheless, DMOG could significantly increase their levels. However, EPA inhibited the effects of DMOG on sFlt-1 mRNA expression (P = 0.0361; Figure 3).
JEG-3 cells treated for 24 hours with eicosapentaenoic acid (50 µm) and dimethyloxalylglycine (100 µM); total ribonucleic acid extracted from JEG-3 cells and messenger ribonucleic acid levels of sFlt-1 analyzed by real-time reverse transcription polymerase chain reaction; values normalized by glyceraldehyde-3-phosphate dehydrogenase as an internal reference; all results represented as mean ± standard deviation from triplicate determinations, representative of three independent experiments compared to control; significant differences between treatments indicated by one-way analysis of variance, followed by Tukey’s multiple comparison test (P < 0.05).
5. Discussion
Omega-3 fatty acids, such as EPA, docosahexaenoic acid (DHA), and alpha-linolenic, are considered essential fatty acids (23). Mainly, LCPUFAs are involved in the functional regulation of cellular and subcellular membranes. Several explanations have been put forward to explain that the altered placental n-3 LCPUFAs, such as EPA, can decrease the sFlt-1 concentration (20, 24), although the mechanism of decreased sFlt-1 production by these compounds remains elusive.
The findings of different studies hypothesize that n-3 LCPUFAs, such as EPA, affect sFlt-1 gene expression and protein secretion in several ways. Firstly, altered membrane n-3 fatty acids levels can change lipid raft function and increase membrane rigidity and lipid fatty acid combination on cell membrane leading to the release of sFlt-1 into the circulation (24). Secondly, n-3 LCPUFAs affect other aspects of inflammation, such as leukocyte chemotaxis and inflammatory cytokine production (25). Thirdly, n-3 LCPUFAs affect angiogenesis by the negative regulation of the cyclooxygenase-2/PGE2 pathway (26). Fourthly, n-3 LCPUFAs can also inhibit the upregulation of sFlt-1 under a hypoxic condition with HIF-1α increasing (6, 27). Numerous studies demonstrated that hypoxia could increase sFlt-1 expression in vascular endothelial cells, placental villous explants, and cytotrophoblast cultures (20).
In this study, it was shown that reduced oxygenation condition induced by DMOG, a pharmacological inhibitor of prolyl-hydroxylases (10), causes the increased expression of sFlt-1 and HIF-1α in cell culture (JEG-3), and EPA as n-3 fatty acids can inhibit the effect of hypoxia stress. Moreover, it was indicated that sFlt-1 expression under a reduced oxygenation condition might be mediated by HIF-1α.
The results of the present study are in line with the results of a study by Calviello et al. demonstrating an EPA-dependent inhibition of gene expression of HIF-1α in HT-29 cells (26). Some studies implied the benefits of n-3 LCPUFAs in pregnancy (28). N-3 LCPUFAs are used as the major source of energy in the human placenta, and defect in the energy-producing pathway prevents the growth, differentiation, and function of the placenta and fetus (29). It is suggested that the safety and efficacy of DHA as the product of EPA supplementation in pregnant women improve pregnancy outcomes (30). It has been considered that the effects of hypoxia as a major trigger for releasing sFlt-1 are mediated by HIF-1α as a transcription factor (10). With regard to the above-mentioned theories, using n-3 LCPUFAs in vivo might treat the adverse effects of sFlt-1 in preeclampsia patients and improve the disease complications. However, preclinical studies remain to be performed.
5.1. Conclusions
This study investigated the EPA effects as the most important n-3 LCPUFA on sFlt-1 RNA and protein expression in the human placental trophoblast JEG-3 cell line. It was reported that oxygen tension (induced by DMOG) could increase sFlt-1 gene expression and protein secretion. However, sFlt-1 expression within the JEG-3 cells was decreased by EPA. Therefore, it is suggested that EPA decreases the sFlt-1 expression and secretion by DMOG via the activation of the HIF-1α pathway.